M-like K+ currents in type I hair cells and calyx afferent endings of the developing rat utricle
- PMID: 17021181
- PMCID: PMC6674627
- DOI: 10.1523/JNEUROSCI.2596-06.2006
M-like K+ currents in type I hair cells and calyx afferent endings of the developing rat utricle
Abstract
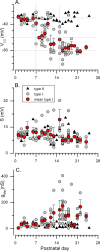
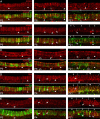
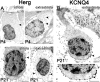
Type I vestibular hair cells have large K+ currents that, like neuronal M currents, activate negative to resting potential and are modulatable. In rodents, these currents are acquired postnatally. In perforated-patch recordings from rat utricular hair cells, immature hair cells [younger than postnatal day 7 (P7)] had a steady-state K+ conductance (g(-30)) with a half-activation voltage (V1/2) of -30 mV. The size and activation range did not change in maturing type II cells, but, by P16, type I cells had added a K conductance that was on average fourfold larger and activated much more negatively. This conductance may comprise two components: g(-60) (V1/2 of -60 mV) and g(-80) (V1/2 of -80 mV). g(-80) washed out during ruptured patch recordings and was blocked by a protein kinase inhibitor. M currents can include contributions from KCNQ and ether-a-go-go-related (erg) channels. KCNQ and erg channel blockers both affected the K+ currents of type I cells, with KCNQ blockers being more potent at younger than P7 and erg blockers more potent at older than P16. Single-cell reverse transcription-PCR and immunocytochemistry showed expression of KCNQ and erg subunits. We propose that KCNQ channels contribute to g(-30) and g(-60) and erg subunits contribute to g(-80). Type I hair cells are contacted by calyceal afferent endings. Recordings from dissociated calyces and afferent endings revealed large K+ conductances, including a KCNQ conductance. Calyx endings were strongly labeled by KCNQ4 and erg1 antisera. Thus, both hair cells and calyx endings have large M-like K+ conductances with the potential to control the gain of transmission.
Figures


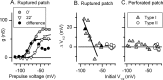








Similar articles
-
Zonal variations in K+ currents in vestibular crista calyx terminals.J Neurophysiol. 2015 Jan 1;113(1):264-76. doi: 10.1152/jn.00399.2014. Epub 2014 Oct 15. J Neurophysiol. 2015. PMID: 25343781 Free PMC article.
-
Resting potential and submembrane calcium concentration of inner hair cells in the isolated mouse cochlea are set by KCNQ-type potassium channels.J Neurosci. 2003 Mar 15;23(6):2141-9. doi: 10.1523/JNEUROSCI.23-06-02141.2003. J Neurosci. 2003. PMID: 12657673 Free PMC article.
-
Voltage-gated calcium channel currents in type I and type II hair cells isolated from the rat crista.J Neurophysiol. 2003 Jul;90(1):155-64. doi: 10.1152/jn.00244.2003. J Neurophysiol. 2003. PMID: 12843307
-
Channeling your inner ear potassium: K(+) channels in vestibular hair cells.Hear Res. 2016 Aug;338:40-51. doi: 10.1016/j.heares.2016.01.015. Epub 2016 Feb 4. Hear Res. 2016. PMID: 26836968 Review.
-
Hair cells in mammalian utricles.Otolaryngol Head Neck Surg. 1998 Sep;119(3):172-81. doi: 10.1016/S0194-5998(98)70052-X. Otolaryngol Head Neck Surg. 1998. PMID: 9743073 Review.
Cited by
-
K+ Accumulation and Clearance in the Calyx Synaptic Cleft of Type I Mouse Vestibular Hair Cells.Neuroscience. 2020 Feb 1;426:69-86. doi: 10.1016/j.neuroscience.2019.11.028. Epub 2019 Dec 14. Neuroscience. 2020. PMID: 31846752 Free PMC article.
-
Development and regeneration of vestibular hair cells in mammals.Semin Cell Dev Biol. 2017 May;65:96-105. doi: 10.1016/j.semcdb.2016.11.001. Epub 2016 Nov 15. Semin Cell Dev Biol. 2017. PMID: 27864084 Free PMC article. Review.
-
Coordinated Expression of Two Types of Low-Threshold K+ Channels Establishes Unique Single Spiking of Mauthner Cells among Segmentally Homologous Neurons in the Zebrafish Hindbrain.eNeuro. 2017 Oct 23;4(5):ENEURO.0249-17.2017. doi: 10.1523/ENEURO.0249-17.2017. eCollection 2017 Sep-Oct. eNeuro. 2017. PMID: 29085904 Free PMC article.
-
The potassium channel subunit KV1.8 (Kcna10) is essential for the distinctive outwardly rectifying conductances of type I and II vestibular hair cells.bioRxiv [Preprint]. 2024 Aug 18:2023.11.21.563853. doi: 10.1101/2023.11.21.563853. bioRxiv. 2024. Update in: Elife. 2024 Dec 03;13:RP94342. doi: 10.7554/eLife.94342. PMID: 38045305 Free PMC article. Updated. Preprint.
-
Postnatal expression of an apamin-sensitive k(ca) current in vestibular calyx terminals.J Membr Biol. 2011 Nov;244(2):81-91. doi: 10.1007/s00232-011-9400-8. Epub 2011 Nov 5. J Membr Biol. 2011. PMID: 22057903 Free PMC article.
References
-
- Anantharam A, Markowitz SM, Abbott GW. Pharmacogenetic considerations in diseases of cardiac ion channels. J Pharmacol Exp Ther. 2003;307:831–838. - PubMed
-
- Barry PH. JPCalc, a software package for calculating liquid junction potential corrections in patch-clamp, intracellular, epithelial and bilayer measurements and for correcting junction potential measurements. J Neurosci Methods. 1994;51:107–116. - PubMed
-
- Brichta AM, Aubert A, Eatock RA, Goldberg JM. Regional analysis of whole cell currents from hair cells of the turtle posterior crista. J Neurophysiol. 2002;88:3259–3278. - PubMed
-
- Brown AM. hERG block, QT liability and sudden cardiac death. In: Chadwick DJ, Goode J, editors. The hERG cardiac potassium channel: structure, function and Long QT syndrome. Chichester, UK: Wiley; 2005. pp. 118–135. - PubMed
-
- Brown DA, Adams PR. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980;283:673–676. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
