Establishment of a rodent model of HIV-associated sensory neuropathy
- PMID: 17021185
- PMCID: PMC6674617
- DOI: 10.1523/JNEUROSCI.3135-06.2006
Establishment of a rodent model of HIV-associated sensory neuropathy
Abstract
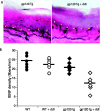
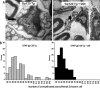
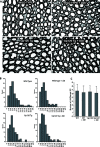
Human immunodeficiency virus (HIV)-associated sensory neuropathy (SN) is the most common neurological complication of HIV infection in the current highly active antiretroviral therapy era. The painful sensory neuropathy is associated with the use of dideoxynucleoside antiretrovirals, and its development limits the choice of antiretroviral drugs in affected patients. There are presently no effective therapies for HIV-SN, and moreover there has been no robust animal model of HIV-SN in which candidate therapeutic agents can be tested. In this paper, we show that we have established a rodent model of HIV-SN by oral administration of a dideoxynucleoside drug, didanosine, to transgenic mice expressing the HIV coat protein gp120 under a GFAP promoter. The neuropathy in these rodents is characterized by distal degeneration of unmyelinated sensory axons, similar to the "dying back" pattern of C-fiber loss seen in patients with HIV-SN. This model will be useful in examining mechanisms of distal axonal degeneration and testing potential neuroprotective compounds that may prevent development of the sensory neuropathy.
Figures

References
-
- Anderson TD, Davidovich A, Feldman D, Sprinkle TJ, Arezzo J, Brosnan C, Calderon RO, Fossom LH, DeVries JT, DeVries GH. Mitochondrial schwannopathy and peripheral myelinopathy in a rabbit model of dideoxycytidine neurotoxicity. Lab Invest. 1994;70:724–739. - PubMed
-
- Apostolski S, McAlarney T, Quattrini A, Levison SW, Rosoklija G, Lugaressi A, Corbo M, Sadiq SA, Lederman S, Hays AP. The gp120 glycoprotein of human immunodeficiency virus type 1 binds to sensory ganglion neurons. Ann Neurol. 1993;34:855–863. - PubMed
-
- Cherry CL, Gahan ME, McArthur JC, Lewin SR, Hoy JF, Wesselingh SL. Exposure to dideoxynucleosides is reflected in lowered mitochondrial DNA in subcutaneous fat. J Acquir Immune Defic Syndr. 2002;30:271–277. - PubMed
-
- Cooney DA, Dalal M, Mitsuya H, McMahon JB, Nadkarni M, Balzarini J, Broder S, Johns DG. Initial studies on the cellular pharmacology of 2′,3-dideoxycytidine, an inhibitor of HTLV-III infectivity. Biochem Pharmacol. 1986;35:2065–2068. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
