Endopeptidase and glycosidase activities of the bacteriophage B30 lysin
- PMID: 17021237
- PMCID: PMC1610294
- DOI: 10.1128/AEM.00829-06
Endopeptidase and glycosidase activities of the bacteriophage B30 lysin
Abstract
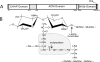
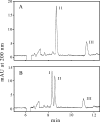
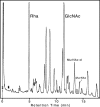
Synthetic peptides corresponding to portions of group B streptococcal peptidoglycan were used to show that the endopeptidase activity of bacteriophage B30 lysin cleaves between D-Ala in the stem peptide and L-Ala in the cross bridge and that the minimal peptide sequence cleaved is DL-gamma-Glu-Lys-D-Ala-Ala-Ala. The only glycosidase activity present is that of N-acetyl-beta-D-muramidase.
Figures
References
-
- Bateman, A., and N. D. Rawlings. 2003. The CHAP domain: a large family of amidases including GSP amidase and peptidoglycan hydrolases. Trends Biochem. Sci. 28:234-237. - PubMed
-
- Carney, S. L., and D. J. Osborne. 1991. The separation of chondroitin sulfate disaccharides and hyaluronan oligosaccharides by capillary zone electrophoresis. Anal. Biochem. 195:132-140. - PubMed
-
- Donovan, D. M., J. Foster-Frey, S. Dong, G. M. Rousseau, S. Moineau, and D. G. Pritchard. 2006. The cell lysis activity of the Streptococcus agalactiae bacteriophage B30 endolysin relies on the cysteine, histidine-dependent amidohydrolase/peptidase domain. Appl. Environ. Microbiol. 72:5108-5112. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

