Hormonal therapy for prostate cancer
Abstract
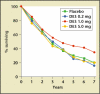
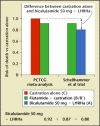
Updates on hormonal therapy in the treatment of prostate cancer are presented. The most common therapy is to reduce testosterone to castrate levels. A dosage of 1 mg diethylstilbestrol daily prolonged survival in patients with advanced prostate cancer. The leuteinizing hormone-releasing hormone agonists have essentially replaced surgical orchiectomy in the vast majority of clinical settings; however, a major problem with the leuteinizing hormone- releasing hormone agonists has been the surge and flare of testosterone levels. If hormonal therapy is initiated early, the risk of major complications is significantly decreased. Combined androgen blockade is better than monotherapy, although there is only a small clinical benefit. When androgen deprivation is used for a short time and the normal androgen milieu is re-established, the side effects and toxicity of androgen deprivation are decreased. The major complications of androgen deprivation include hot flushes, reduction of bone mineral density, osteoporosis, and anemia. Intermittent androgen blockade might have the same benefits of total androgen suppression with fewer side effects, increased duration of androgen dependence, and less cost. The 10 steps to take when advising patients about initiation of androgen deprivation therapy are reviewed.
Figures

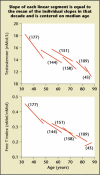

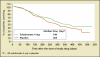
References
-
- Huggins C, Hodges CV. Studies on prostatic cancer I. The effect of castration, estrogen, and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293–297.
-
- Byar DP, Corle DK. Hormone therapy for prostate cancer: results of the Veterans Administration Cooperative Urological Research Group studies. NCI Monogr. 1988;7:165–170. - PubMed
-
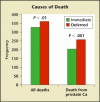
- Immediate versus deferred treatment for advanced prostatic cancer: initial results of the Medical Research Council Trial. The Medical Research Council Prostate Cancer Working Party Investigators Group. Br J Urol. 1997;79:235–246. - PubMed
-
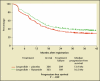
- Pilepich MV, Winter K, Lawton CA, et al. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma—long-term results of phase III RTOG 85-31. Int J Radiat Oncol Biol Phys. 2005;61:1285–1290. - PubMed
-
- Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002;360:103–106. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
