The incidence of arthropathy adverse events in efalizumab-treated patients is low and similar to placebo and does not increase with long-term treatment: pooled analysis of data from Phase III clinical trials of efalizumab
- PMID: 17021768
- PMCID: PMC1705535
- DOI: 10.1007/s00403-006-0694-9
The incidence of arthropathy adverse events in efalizumab-treated patients is low and similar to placebo and does not increase with long-term treatment: pooled analysis of data from Phase III clinical trials of efalizumab
Abstract
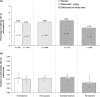
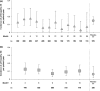
A large-scale, pooled analysis of safety data from five Phase III clinical trials (including open-label extensions of two of these studies) and two Phase III open-label clinical trials of efalizumab was conducted to explore whether arthropathy adverse events (AEs) were associated with efalizumab treatment in patients with moderate-to-severe chronic plaque psoriasis. Data from patients who received subcutaneous injections of efalizumab or placebo were stratified for analysis into phases according to the nature and duration of treatment. These included: the 'first treatment' phase (0-12-week data from patients who received either efalizumab, 1 mg/kg once weekly, or placebo in the five placebo-controlled studies); the 'extended treatment' phase (13-24-week data from seven trials for all efalizumab-treated patients); and the 'long-term treatment' phase (data from efalizumab-treated patients who received treatment for up to 36 months in two long-term trials). Descriptive statistics were performed and the incidence of arthropathy AEs per patient-year was calculated using 95% confidence intervals (CIs). During the first treatment phase, a similar proportion of patients had an arthropathy AE in the efalizumab group (3.3%; 58/1740 patients) compared with the placebo group (3.5%; 34/979 patients); the incidence of arthropathy AEs per patient-year was 0.15 in the efalizumab group (95% CI 0.11-0.19) and 0.16 in the placebo group (95% CI 0.11-0.22). Analysis of first treatment phase data from one study (n = 793) showed that the incidence of psoriatic arthropathy per patient-year was lower in efalizumab-treated patients (0.10; 95% CI 0.05-0.18) than in those given placebo (0.17; 95% CI 0.08-0.30). During the extended treatment phase, the incidence of arthropathy remained low (0.17; 95% CI 0.14-0.22). Data from two long-term studies showed that there was no increase in the incidence of arthropathy AEs over time in patients treated with efalizumab for up to 36 months. Patients who had an arthropathy AE during treatment with efalizumab appeared to be more likely to have a history of arthropathy prior to treatment. Efalizumab does not appear to increase the risk of arthropathy AEs compared with placebo.
Figures
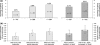
References
-
- EMEA (2000) EMEA public statement on infliximab (Remicade)—reports of tuberculosis infections. Accessed 17 January 2006 http://www.emea.eu.int/pdfs/human/press/pus/444500en.pdf
-
- FDA (1995) COSTART: coding symbols for thesaurus of adverse reaction terms, 5th edn. pp 1–540
-
- Gottlieb AB, Gordon KB, Hamilton TK, Caro I, Kwon P, Compton P (2005) Maintenance of efficacy and safety with continuous efalizumab therapy in patients with moderate to severe chronic plaque psoriasis: final phase IIIb study results. Poster presented at the 63rd Annual Meeting of the American Academy of Dematology (AAD)
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

