Immune suppression of challenged vaccinates as a rigorous assessment of sterile protection by lentiviral vaccines
- PMID: 17023099
- PMCID: PMC1855206
- DOI: 10.1016/j.vaccine.2006.09.040
Immune suppression of challenged vaccinates as a rigorous assessment of sterile protection by lentiviral vaccines
Abstract
We previously reported that an experimental live-attenuated equine infectious anemia virus (EIAV) vaccine, containing a mutated S2 accessory gene, provided protection from disease and detectable infection after virulent virus (EIAV(PV)) challenge [Li F, Craigo JK, Howe L, Steckbeck JD, Cook S, Issel C, et al. A live-attenuated equine infectious anemia virus proviral vaccine with a modified S2 gene provides protection from detectable infection by intravenous virulent virus challenge of experimentally inoculated horses. J Virol 2003;77(13):7244-53; Craigo JK, Li F, Steckbeck JD, Durkin S, Howe L, Cook SJ, et al. Discerning an effective balance between equine infectious anemia virus attenuation and vaccine efficacy. J Virol 2005;79(5):2666-77]. To determine if attenuated EIAV vaccines actually prevent persistent infection by challenge virus, we employed a 14-day dexamethasone treatment of vaccinated horses post-challenge to suppress host immunity and amplify replication levels of any infecting EIAV. At 2 months post-challenge the horses were all protected from virulent-virus challenge, evidenced by a lack of EIA signs and detectable challenge plasma viral RNA. Upon immune suppression, 6/12 horses displayed clinical EIA. Post-immune suppression characterizations demonstrated that the attenuated vaccine evidently prevented detectable challenge virus infection in 50% of horses. These data highlight the utility of post-challenge immune suppression for evaluating persistent viral vaccine protective efficacy.
Figures


 , right Y axis) and platelet counts (
, right Y axis) and platelet counts (
 , first left Y axis) were followed daily for up to 300 days (X-axis) after the first vaccine dose. Quantification of the virus load (
, first left Y axis) were followed daily for up to 300 days (X-axis) after the first vaccine dose. Quantification of the virus load (
 , second left Y axis) was performed on viral RNA extracted from plasma at periodic time points prior to and after virulent virus challenge using the LDME protocol (DOC,↓↓↓). The period of dexamethasone-induced immune suppression is demarcated by a pink shaded box. Two naïve control animals (Panels M-N) were also challenged with the LDME protocol (DOC,↓↓↓). Febrile episodes were defined by a rectal temperature above 39°C (
, second left Y axis) was performed on viral RNA extracted from plasma at periodic time points prior to and after virulent virus challenge using the LDME protocol (DOC,↓↓↓). The period of dexamethasone-induced immune suppression is demarcated by a pink shaded box. Two naïve control animals (Panels M-N) were also challenged with the LDME protocol (DOC,↓↓↓). Febrile episodes were defined by a rectal temperature above 39°C (
 ) in conjunction with thrombocytopenia (platelets ≤100,000/μl of whole blood) and other clinical symptoms of EIA. S2 diagnostic results for each animal are indicated in each respective panel above the date of analysis by either a V (vaccine strain) or a C (challenge strain).
) in conjunction with thrombocytopenia (platelets ≤100,000/μl of whole blood) and other clinical symptoms of EIA. S2 diagnostic results for each animal are indicated in each respective panel above the date of analysis by either a V (vaccine strain) or a C (challenge strain).
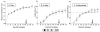
 ).
).
References
-
- Li F, Craigo JK, Howe L, Steckbeck JD, Cook S, Issel C, et al. A Live Attenuated Equine Infectious Anemia Virus Proviral Vaccine with a Modified S2 Gene Provides Protection from Detectable Infection by Intravenous Virulent Virus Challenge of Experimentally Inoculated Horses. The Journal of Virology. 2003;77(13):7244–7253. - PMC - PubMed
-
- Mims CA. The Pathogenesis of Infectious Diseases. 3. SanDiego: Academic; 1987.
-
- Melink JA. Vaccines. 2. SanDiego: Academic; 1994.
-
- Baba TW, Liska V, Khimani AH, Ray NB, Dailey PJ, Penninck D, et al. Live attenuated, multiply deleted simian immunodeficiency virus causes AIDS in infant and adult macaques. Nat Med. 1999;5:194–203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

