A small molecule agonist of an integrin, alphaLbeta2
- PMID: 17023419
- PMCID: PMC1764823
- DOI: 10.1074/jbc.M606888200
A small molecule agonist of an integrin, alphaLbeta2
Abstract
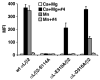
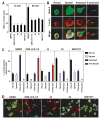
The binding of integrin alpha(L)beta(2) to its ligand intercellular adhesion molecule-1 is required for immune responses and leukocyte trafficking. Small molecule antagonists of alpha(L)beta(2) are under intense investigation as potential anti-inflammatory drugs. We describe for the first time a small molecule integrin agonist. A previously described alpha/beta I allosteric inhibitor, compound 4, functions as an agonist of alpha(L)beta(2) in Ca(2+) and Mg(2+)and as an antagonist in Mn(2+). We have characterized the mechanism of activation and its competitive and noncompetitive inhibition by different compounds. Although it stimulates ligand binding, compound 4 nonetheless inhibits lymphocyte transendothelial migration. Agonism by compound 4 results in accumulation of alpha(L)beta(2) in the uropod, extreme uropod elongation, and defective de-adhesion. Small molecule integrin agonists open up novel therapeutic possibilities.
Figures


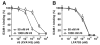


References
-
- Anderson DC, Smith CW, Springer TA. In: Metabolic Basis of Inherited Disease. 6th Ed. Stanbury JB, Wyngaarden JB, Frederickson DS, editors. McGraw-Hill; New York: 1989. pp. 2751–2777.
-
- Springer TA. Nature. 1990;346:425–433. - PubMed
-
- Harlan JM, Winn RK, Vedder NB, Doerschuk CM, Rice CL. In: Adhesion: Its Role in Inflammatory Disease. Harlan JR, Liu D, editors. W. H. Freeman and Co.; New York: 1992. pp. 117–150.
-
- Giblin PA, Kelly TA. Annu Rep Med Chem. 2001;36:181–190.
-
- Yusuf-Makagiansar H, Anderson ME, Yakovleva TV, Murray JS, Siahaan TJ. Med Res Rev. 2002;22:146–167. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

