Effects of flecainide and quinidine on arrhythmogenic properties of Scn5a+/Delta murine hearts modelling long QT syndrome 3
- PMID: 17023504
- PMCID: PMC1810389
- DOI: 10.1113/jphysiol.2006.117945
Effects of flecainide and quinidine on arrhythmogenic properties of Scn5a+/Delta murine hearts modelling long QT syndrome 3
Abstract
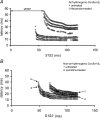
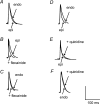
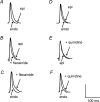
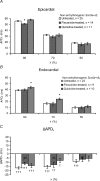
Long QT3 (LQT3) syndrome is associated with incomplete Na+ channel inactivation, abnormal repolarization kinetics and prolonged cardiac action potential duration (APD). Electrophysiological effects of flecainide and quinidine were compared in Langendorff-perfused wild-type (WT), and genetically modified (Scn5a+/Delta) murine hearts modelling LQT3. Extra stimuli (S2) following trains of pacing stimuli (S1) applied to the right ventricular epicardium triggered ventricular tachycardia (VT) in 16 out of 28 untreated Scn5a+/Delta and zero out of 12 WT hearts. Paced electrogram fractionation analysis then demonstrated increased electrogram durations (EGD), expressed as EGD ratios, in arrhythmogenic Scn5a+/Delta hearts, and prolonged ventricular effective refractory periods in initially non-arrhythmogenic Scn5a+/Delta hearts. Nevertheless, comparisons of epicardial and endocardial monophasic action potential recordings demonstrated negative transmural repolarization gradients in both groups, giving DeltaAPD(90) values at 90% repolarization of -20.88 +/- 1.93 ms (n = 11) and -16.91 +/- 1.43 ms (n = 23), respectively. Flecainide prevented initiation of VT in 13 out of 16 arrhythmogenic Scn5a+/Delta hearts, reducing EGD ratio and restoring DeltaAPD90 to + 7.55 +/- 2.24 ms (n = 9) (P < 0.05). VT occurred in four out of eight non-arrhythmogenic Scn5a+/Delta hearts in the presence of quinidine, which increased EGD ratio but left DeltaAPD90 unchanged. In contrast (P < 0.05), WT hearts had positive DeltaAPD90 values (+ 11.72 +/- 2.17 ms) (n = 20). Flecainide then increased arrhythmic tendency and EGD ratio but conserved DeltaAPD90; reduced EGD ratios and unaltered DeltaAPD90 values accompanied the lower arrhythmogenicity associated with quinidine treatment. In addition to the changes in EGD ratio shown by WT hearts, these findings attribute arrhythmogenesis and its modification by flecainide and quinidine to alterations in DeltaAPD90 in Scn5a+/Delta hearts. This is consistent with a hypothesis in which incomplete Na+ channel inactivation in Scn5a+/Delta hearts generates functional substrates dependent on altered refractoriness that cause abnormalities in activation and conduction of subsequent cardiac impulses. Any spatial heterogeneities between the epicardial and endocardial layers would thus cause fragmentation of the activation wavefront and contribute to electrogram spreading.
Figures




References
-
- Abriel H, Wehrens XH, Benhorin J, Kerem B, Kass RS. Molecular pharmacology of the sodium channel mutation D1790G linked to the long–QT syndrome. Circulation. 2000;102:921–925. - PubMed
-
- Alings M, Dekker L, Sadee A, Wilde A. Quinidine-induced electrocardiographic normalization in two patients with Brugada syndrome. Pacing Clin Electrophysiol. 2001;24:1420–1422. - PubMed
-
- Alings M, Wilde A. ‘Brugada’ syndrome: clinical data and suggested pathophysiological mechanism. Circulation. 1999;99:666–673. - PubMed
-
- Antzelevitch C, Dumaine R. Electrical heterogeneity in the heart: physiological, pharmacological and clinical implication. In: Page E, Fozzard HA, Solaro RJ, editors. Handbook of Physiology. New York: Oxford University Press; 2001. pp. 654–692.
-
- Antzelevtich C, Fish J. Electrical heterogeneity within the ventricular wall. Basic Res Cardiol. 2001;96:517–527. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

