Recent progress in understanding hydrophobic interactions
- PMID: 17023540
- PMCID: PMC1635073
- DOI: 10.1073/pnas.0606422103
Recent progress in understanding hydrophobic interactions
Abstract
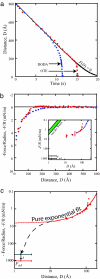
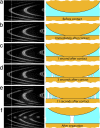
We present here a brief review of direct force measurements between hydrophobic surfaces in aqueous solutions. For almost 70 years, researchers have attempted to understand the hydrophobic effect (the low solubility of hydrophobic solutes in water) and the hydrophobic interaction or force (the unusually strong attraction of hydrophobic surfaces and groups in water). After many years of research into how hydrophobic interactions affect the thermodynamic properties of processes such as micelle formation (self-assembly) and protein folding, the results of direct force measurements between macroscopic surfaces began to appear in the 1980s. Reported ranges of the attraction between variously prepared hydrophobic surfaces in water grew from the initially reported value of 80-100 Angstrom to values as large as 3,000 Angstrom. Recent improved surface preparation techniques and the combination of surface force apparatus measurements with atomic force microscopy imaging have made it possible to explain the long-range part of this interaction (at separations >200 Angstrom) that is observed between certain surfaces. We tentatively conclude that only the short-range part of the attraction (<100 Angstrom) represents the true hydrophobic interaction, although a quantitative explanation for this interaction will require additional research. Although our force-measuring technique did not allow collection of reliable data at separations <10 Angstrom, it is clear that some stronger force must act in this regime if the measured interaction energy curve is to extrapolate to the measured adhesion energy as the surface separation approaches zero (i.e., as the surfaces come into molecular contact).
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Comment in
-
Profile of Jacob N. Israelachvili.Proc Natl Acad Sci U S A. 2006 Oct 24;103(43):15736-8. doi: 10.1073/pnas.0607766103. Epub 2006 Oct 16. Proc Natl Acad Sci U S A. 2006. PMID: 17043213 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

