Urea stimulation of KCl cotransport induces abnormal volume reduction in sickle reticulocytes
- PMID: 17023583
- PMCID: PMC1794068
- DOI: 10.1182/blood-2006-04-018630
Urea stimulation of KCl cotransport induces abnormal volume reduction in sickle reticulocytes
Erratum in
- Blood. 2007 Apr 1;109(7):2735
Abstract
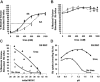
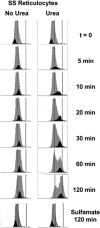
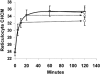
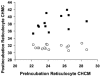
KCl cotransport (KCC) activity contributes to pathologic dehydration in sickle (SS) red blood cells (RBCs). KCC activation by urea was measured in SS and normal (AA) RBCs as Cl-dependent Rb influx. KCC-mediated volume reduction was assessed by measuring reticulocyte cellular hemoglobin concentration (CHC) cytometrically. Urea activated KCC fluxes in fresh RBCs to levels seen in swollen cells, although SS RBCs required lower urea concentrations than did normal (AA) RBCs. Little additional KCC stimulation by urea occurred in swollen AA or SS RBCs. The pH dependence of KCC in "euvolemic" SS RBCs treated with urea was similar to that in swollen cells. Urea triggered volume reduction in SS and AA reticulocytes, establishing a higher CHC. Volume reduction was Cl dependent and was limited by the KCC inhibitor, dihydro-indenyl-oxyalkanoic acid. Final CHC depended on urea concentration, but not on initial CHC. Under all activation conditions, volume reduction was exaggerated in SS reticulocytes and produced higher CHCs than in AA reticulocytes. The sulfhydryl-reducing agent, dithiothreitol, normalized the sensitivity of KCC activation to urea in SS RBCs and mitigated the urea-stimulated volume decrease in SS reticulocytes, suggesting that the dysfunctional activity of KCC in SS RBCs was due in part to reversible sulfhydryl oxidation.
Figures
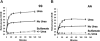

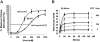
References
-
- Adragna NC, Fulvio MD, Lauf PK. Regulation of K-Cl cotransport: from function to genes. J Membr Biol. 2004;201:109–137. - PubMed
-
- Gamba G. Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters. Physiol Rev. 2005;85:423–493. - PubMed
-
- Bize I, Taher S, Brugnara C. Regulation of K-Cl cotransport during reticulocyte maturation and erythrocyte aging in normal and sickle erythrocytes. Am J Physiol Cell Physiol. 2003;285:C31–C38. - PubMed
-
- Canessa M, Fabry ME, Blumenfeld N, Nagel RL. Volume-stimulated, Cl(-)-dependent K+ efflux is highly expressed in young human red cells containing normal hemoglobin or HbS. J Membr Biol. 1987;97:97–105. - PubMed
-
- Olivieri O, Vitoux D, Galacteros F, et al. Hemoglobin variants and activity of the (K+Cl-) cotransport system in human erythrocytes. Blood. 1992;79:793–797. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

