Deletion of tetraspanin Cd151 results in decreased pathologic angiogenesis in vivo and in vitro
- PMID: 17023588
- PMCID: PMC1794066
- DOI: 10.1182/blood-2006-08-041970
Deletion of tetraspanin Cd151 results in decreased pathologic angiogenesis in vivo and in vitro
Abstract
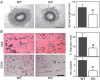
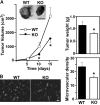
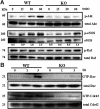
Tetraspanin protein CD151 is abundant on endothelial cells. To determine whether CD151 affects angiogenesis, Cd151-null mice were prepared. Cd151-null mice showed no vascular defects during normal development or during neonatal oxygen-induced retinopathy. However, Cd151-null mice showed impaired pathologic angiogenesis in other in vivo assays (Matrigel plug, corneal micropocket, tumor implantation) and in the ex vivo aortic ring assay. Cd151-null mouse lung endothelial cells (MLECs) showed normal adhesion and proliferation, but marked alterations in vitro, in assays relevant to angiogenesis (migration, spreading, invasion, Matrigel contraction, tube and cable formation, spheroid sprouting). Consistent with these functional impairments, and with the close, preferential association of CD151 with laminin-binding integrins, Cd151-null MLECs also showed selective signaling defects, particularly on laminin substrate. Adhesion-dependent activation of PKB/c-Akt, e-NOS, Rac, and Cdc42 was diminished, but Raf, ERK, p38 MAP kinase, FAK, and Src were unaltered. In Cd151-null MLECs, connections were disrupted between laminin-binding integrins and at least 5 other proteins. In conclusion, CD151 modulates molecular organization of laminin-binding integrins, thereby supporting secondary (ie, after cell adhesion) functions of endothelial cells, which are needed for some types of pathologic angiogenesis in vivo. Selective effects of CD151 on pathologic angiogenesis make it a potentially useful target for anticancer therapy.
Figures




References
-
- Dvorak HF. Angiogenesis: update 2005. J Thromb Haemost. 2005;3:1835–1842. - PubMed
-
- Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. - PubMed
-
- Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. - PubMed
-
- Stupack DG, Cheresh DA. Integrins and angiogenesis. Curr Top Dev Biol. 2004;64:207–238. - PubMed
-
- Hynes RO. A reevaluation of integrins as regulators of angiogenesis. Nat Med. 2002;8:918–921. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

