Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior
- PMID: 17023662
- PMCID: PMC1880880
- DOI: 10.1126/science.1129663
Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior
Abstract
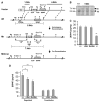
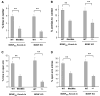
A common single-nucleotide polymorphism in the brain-derived neurotrophic factor (BDNF) gene, a methionine (Met) substitution for valine (Val) at codon 66 (Val66Met), is associated with alterations in brain anatomy and memory, but its relevance to clinical disorders is unclear. We generated a variant BDNF mouse (BDNF(Met/Met)) that reproduces the phenotypic hallmarks in humans with the variant allele. BDNF(Met) was expressed in brain at normal levels, but its secretion from neurons was defective. When placed in stressful settings, BDNF(Met/Met) mice exhibited increased anxiety-related behaviors that were not normalized by the antidepressant, fluoxetine. A variant BDNF may thus play a key role in genetic predispositions to anxiety and depressive disorders.
Figures

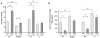
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

