Injection of the sciatic nerve with TMEV: a new model for peripheral nerve demyelination
- PMID: 17028060
- PMCID: PMC1847644
- DOI: 10.1016/j.virol.2006.09.009
Injection of the sciatic nerve with TMEV: a new model for peripheral nerve demyelination
Abstract
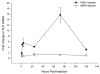
Demyelination of the human peripheral nervous system (PNS) can be caused by diverse mechanisms including viral infection. Despite association of several viruses with the development of peripheral demyelination, animal models of the condition have been limited to disease that is either autoimmune or genetic in origin. We describe here a model of PNS demyelination based on direct injection of sciatic nerves of mice with the cardiovirus, Theiler's murine encephalomyelitis virus (TMEV). Sciatic nerves of FVB mice develop inflammatory cell infiltration following TMEV injection. Schwann cells and macrophages are infected with TMEV. Viral replication is observed initially in the sciatic nerves and subsequently the spinal cord. Sciatic nerves are demyelinated by day 5 post-inoculation (p.i.). Injecting sciatic nerves of scid mice resulted in increased levels of virus recovered from the sciatic nerve and spinal cord relative to FVB mice. Demyelination also occurred in scid mice and by 12 days p.i., hindlimbs were paralyzed. This new model of virus-induced peripheral demyelination may be used to dissect processes involved in protection of the PNS from viral insult and to study the early phases of lesion development.
Figures




References
-
- Bernhardt G, Bibb JA, Bradley J, Wimmer E. Molecular characterization of the cellular receptor for poliovirus. Virol. 1994;199:105–113. - PubMed
-
- Bitan M, Or R, Shapira MY, Mador N, Resnick IB, Saleh N, Weinberger KM, Samuel S, Schechter E, Slavin S, Wolf DG. Early-onset Guillain-Barre syndrome associated with reactivation of Epstein-Barr virus infection after nonmyeloablative stem cell transplantation. Clin Infect Dis. 2004;39:1076–1078. - PubMed
-
- Bosma GC, Custer RP, Bosma MJ. A severe combined immunodeficiency mutation in the mouse. Nature. 1983;301:527–530. - PubMed
-
- Bour-Jordan H, Thompson HL, Bluestone JA. Distinct effector mechanisms in the development of autoimmune neuropathy versus diabetes in nonobese diabetic mice. J Immunol. 2005;175:5649–5655. - PubMed
-
- Brannagan TH, 3, Zhou Y. HIV-associated Guillain-Barre syndrome. J Neurol Sci. 2003;208:39–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

