Contour length and refolding rate of a small protein controlled by engineered disulfide bonds
- PMID: 17028145
- PMCID: PMC1697845
- DOI: 10.1529/biophysj.106.091561
Contour length and refolding rate of a small protein controlled by engineered disulfide bonds
Abstract
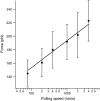
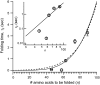
The introduction of disulfide bonds into proteins creates additional mechanical barriers and limits the unfolded contour length (i.e., the maximal extension) measured by single-molecule force spectroscopy. Here, we engineer single disulfide bonds into four different locations of the human cardiac titin module (I27) to control the contour length while keeping the distance to the transition state unchanged. This enables the study of several biologically important parameters. First, we are able to precisely determine the end-to-end length of the transition state before unfolding (53 Angstrom), which is longer than the end-to-end length of the protein obtained from NMR spectroscopy (43 Angstrom). Second, the measured contour length per amino acid from five different methods (4.0 +/- 0.2 Angstrom) is longer than the end-to-end length obtained from the crystal structure (3.6 Angstrom). Our measurement of the contour length takes into account all the internal degrees of freedom of the polypeptide chain, whereas crystallography measures the end-to-end length within the "frozen" protein structure. Furthermore, the control of contour length and therefore the number of amino acids unraveled before reaching the disulfide bond (n) facilitates the test of the chain length dependence on the folding time (tau(F)). We find that both a power law scaling tau(F) lambda n(lambda) with lambda = 4.4, and an exponential scaling with n(0.6) fit the data range, in support of different protein-folding scenarios.
Figures


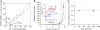
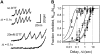
References
-
- Bustamante, C., J. F. Marko, E. D. Siggia, and S. Smith. 1994. Entropic elasticity of lambda-phage DNA. Science. 265:1599–1600. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

