Loading of Arabidopsis centromeric histone CENH3 occurs mainly during G2 and requires the presence of the histone fold domain
- PMID: 17028205
- PMCID: PMC1626606
- DOI: 10.1105/tpc.106.043174
Loading of Arabidopsis centromeric histone CENH3 occurs mainly during G2 and requires the presence of the histone fold domain
Abstract
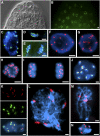
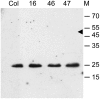
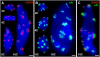
The centromeric histone H3 (CENH3) substitutes histone H3 within the nucleosomes of active centromeres in all eukaryotes. CENH3 deposition at centromeres is needed to assemble the kinetochore, a complex of conserved proteins responsible for correct chromosome segregation during nuclear division. Histones of regular nucleosomes are loaded during replication in S phase, while CENH3 deposition deviates from this pattern in yeast, human, and Drosophila melanogaster cells. Little is known about when and how CENH3 targets centromeric loci. Therefore, we determined the location and quantity of recombinant enhanced yellow fluorescent protein (EYFP)-CENH3 in mitotic root and endopolyploid leaf nuclei of transgenic Arabidopsis thaliana cells. Our data indicate significant loading of A. thaliana CENH3 during G2 (before splitting into sister kinetochores) rather than during the S or M phase of the cell cycle. The histone fold domain of the C-terminal part of CENH3 is sufficient to target A. thaliana centromeres. A. thaliana EYFP-CENH3 can recognize and target three different centromeric repeats of Arabidopsis lyrata but not field bean (Vicia faba) centromeres.
Figures
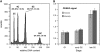
References
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

