Chick integrin alpha V subunit molecular analysis reveals high conservation of structural domains and association with multiple beta subunits in embryo fibroblasts
- PMID: 1703004
- PMCID: PMC2758227
- DOI: 10.1021/bi00496a006
Chick integrin alpha V subunit molecular analysis reveals high conservation of structural domains and association with multiple beta subunits in embryo fibroblasts
Abstract
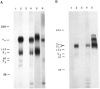
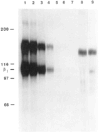
We have cloned and characterized a chick homologue of the human vitronectin receptor alpha subunit (alpha v) whose primary sequence is 83% identical with its human counterpart but less than 40% identical with any other known integrin alpha subunit. Comparison of the chick and human sequences reveals several highly conserved regions, including the cytoplasmic domain. The putative ligand binding domain contains alpha v-specific residues that may contribute to ligand binding specificity. These are concentrated in three regions that are located before and between the first three Ca2+ binding domains. Polyclonal antibodies raised against two peptides deduced from the putative cytoplasmic and extracellular domains of the chick alpha v sequence recognize specifically integrin heterodimers in chick embryo fibroblasts. At least three putative beta subunits coimmunoprecipitate with the chick alpha v subunit. In addition to a protein with the same molecular weight as beta 3 (94K), protein bands of Mr 84K and 110K are also coprecipitated. By successive immunodepletions, we demonstrate that this latter Mr 110K subunit is beta 1, which appears to be one of the alpha v-associated subunits in chick embryo fibroblasts.
Figures




References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
