The CDM superfamily protein MBC directs myoblast fusion through a mechanism that requires phosphatidylinositol 3,4,5-triphosphate binding but is independent of direct interaction with DCrk
- PMID: 17030600
- PMCID: PMC1698515
- DOI: 10.1128/MCB.00016-06
The CDM superfamily protein MBC directs myoblast fusion through a mechanism that requires phosphatidylinositol 3,4,5-triphosphate binding but is independent of direct interaction with DCrk
Abstract
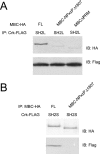
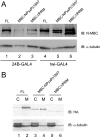
Myoblast city (mbc), a member of the CDM superfamily, is essential in the Drosophila melanogaster embryo for fusion of myoblasts into multinucleate fibers. Using germ line clones in which both maternal and zygotic contributions were eliminated and rescue of the zygotic loss-of-function phenotype, we established that mbc is required in the fusion-competent subset of myoblasts. Along with its close orthologs Dock180 and CED-5, MBC has an SH3 domain at its N terminus, conserved internal domains termed DHR1 and DHR2 (or "Docker"), and C-terminal proline-rich domains that associate with the adapter protein DCrk. The importance of these domains has been evaluated by the ability of MBC mutations and deletions to rescue the mbc loss-of-function muscle phenotype. We demonstrate that the SH3 and Docker domains are essential. Moreover, ethyl methanesulfonate-induced mutations that change amino acids within the MBC Docker domain to residues that are conserved in other CDM family members nevertheless eliminate MBC function in the embryo, which suggests that these sites may mediate interactions specific to Drosophila MBC. A functional requirement for the conserved DHR1 domain, which binds to phosphatidylinositol 3,4,5-triphosphate, implicates phosphoinositide signaling in myoblast fusion. Finally, the proline-rich C-terminal sites mediate strong interactions with DCrk, as expected. These sites are not required for MBC to rescue the muscle loss-of-function phenotype, however, which suggests that MBC's role in myoblast fusion can be carried out independently of direct DCrk binding.
Figures







References
-
- Abmayr, S. M., and K. S. Kocherlakota. 2005. Muscle morphogenesis: the process of embryonic myoblast fusion, p. 92-103. In H. Sink (ed.), Muscle development in Drosophila. Springer Science & Business Media, Inc., New York, N.Y.
-
- Akakura, S., B. Kar, S. Singh, L. Cho, N. Tibrewal, R. Sanokawa-Akakura, C. Reichman, K. S. Ravichandran, and R. B. Birge. 2005. C-terminal SH3 domain of CrkII regulates the assembly and function of the DOCK180/ELMO Rac-GEF. J. Cell. Physiol. 204:344-351. - PubMed
-
- Bourne, H. R. 2005. Rac and cell migration: CDM proteins integrate signals. Nat. Cell Biol. 7:777-778. - PubMed
-
- Brand, A. H., and N. Perrimon. 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118:401-415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
