Cell confluence regulates hepatocyte growth factor-stimulated cell morphogenesis in a beta-catenin-dependent manner
- PMID: 17030602
- PMCID: PMC1698536
- DOI: 10.1128/MCB.01312-06
Cell confluence regulates hepatocyte growth factor-stimulated cell morphogenesis in a beta-catenin-dependent manner
Abstract
Following organ injury, morphogenic epithelial responses can vary depending on local cell density. In the present study, the role of cell confluence in determining the responsiveness of renal epithelial cells to the dedifferentiating morphogenic signals of hepatocyte growth factor (HGF) was examined. Increasing confluence resulted in a greater tendency of cells to organize into epithelial tubes and a significant decrease in migratory responsiveness to HGF. Analysis of downstream signaling revealed that the HGF receptor c-Met was equally activated in confluent and nonconfluent cells following HGF stimulation but that phosphoinositide 3-kinase-dependent activation of Akt and Rac were selectively diminished in confluent cells. In nonconfluent cells treated with HGF, the high level of Akt activation resulted in inhibitory phosphorylation of glycogen synthase kinase 3beta (GSK-3beta) and increased beta-catenin nuclear signaling. In contrast, confluent cells, in which HGF-stimulated Akt activation was diminished, displayed less inhibitory phosphorylation of GSK-3beta and less nuclear signaling by beta-catenin. Overexpression of beta-catenin (SA), which cannot be phosphorylated by GSK-3beta and targeted for ubiquitination, significantly increased migration in fully confluent cells. Thus, cells maintained at high confluence selectively downregulate signaling events such as Rac activation and beta-catenin-dependent transcription that would otherwise promote cell dedifferentiation and migration.
Figures

 , P < 0.01 versus cells at 25,000 cells/well plus HGF;
, P < 0.01 versus cells at 25,000 cells/well plus HGF; 
 , P < 0.01 versus cells at 25,000 or 200,000 cells/well plus HGF.
, P < 0.01 versus cells at 25,000 or 200,000 cells/well plus HGF.

 , P < 0.01 versus nonconfluent cells plus HGF. (C) Quiescent nonconfluent and confluent cells were treated with HGF for the indicated times (in minutes), and cell lysates were immunoblotted with α-pAkt (upper panel) and α-Akt (lower panel). (D) Quiescent nonconfluent cells were pretreated for 20 min in the presence or absence of 10 mM LY294002, then stimulated in the presence or absence of HGF for 10 min, and immunoblotted with α-pAkt (upper panel) and α-Akt (lower panel). (E) Quiescent confluent and nonconfluent mIMCD-3 cells plated for 24 h were stimulated in the presence or absence of HGF (40 ng/ml) for 10 min and immunoblotted with α-pERK (upper panel) and α-ERK (lower panel). (F) Densitometric quantification of four independent experiments was performed as described for panel E.
, P < 0.01 versus nonconfluent cells plus HGF. (C) Quiescent nonconfluent and confluent cells were treated with HGF for the indicated times (in minutes), and cell lysates were immunoblotted with α-pAkt (upper panel) and α-Akt (lower panel). (D) Quiescent nonconfluent cells were pretreated for 20 min in the presence or absence of 10 mM LY294002, then stimulated in the presence or absence of HGF for 10 min, and immunoblotted with α-pAkt (upper panel) and α-Akt (lower panel). (E) Quiescent confluent and nonconfluent mIMCD-3 cells plated for 24 h were stimulated in the presence or absence of HGF (40 ng/ml) for 10 min and immunoblotted with α-pERK (upper panel) and α-ERK (lower panel). (F) Densitometric quantification of four independent experiments was performed as described for panel E.
 , P < 0.01 versus nonconfluent cells plus HGF. (E) Quiescent confluent and nonconfluent mIMCD-3 cells were treated in the presence or absence of HGF, followed by PBD pull-down of GTP-Rac and immunoblotting with α-Rac (upper panel). Whole-cell lysates (WCL) were immunoblotted with α-Rac (detecting both GDP-Rac and GTP-Rac) to determine equality of starting material (lower panel). (F) Densitometric quantification of four independent experiments was performed as described for panel E.
, P < 0.01 versus nonconfluent cells plus HGF. (E) Quiescent confluent and nonconfluent mIMCD-3 cells were treated in the presence or absence of HGF, followed by PBD pull-down of GTP-Rac and immunoblotting with α-Rac (upper panel). Whole-cell lysates (WCL) were immunoblotted with α-Rac (detecting both GDP-Rac and GTP-Rac) to determine equality of starting material (lower panel). (F) Densitometric quantification of four independent experiments was performed as described for panel E.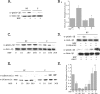
 , P < 0.01 versus nonconfluent cells plus HGF. (C) Quiescent nonconfluent and confluent cells were treated with HGF for the indicated times (minutes), and lysates were immunoblotted with α-pGSK-3β (upper panel) and α-GSK-3β (lower panel). (D) Quiescent nonconfluent cells were pretreated for 20 min with either 10 μM LY294002 or 10 μM Akt IV inhibitor (AktI) and then treated in the presence or absence of HGF for 10 min, and cell lysates were immunoblotted with α-pGSK-3β (upper panel) and α-GSK-3β (lower panel). (E) Quiescent confluent and nonconfluent mIMCD-3 cells were stimulated with HGF for the indicated times and immunoblotted with α-pβ-catenin (upper panel) and α-β-catenin (lower panel). (F) Densitometric quantification of four independent experiments was performed as described for panel E.
, P < 0.01 versus nonconfluent cells plus HGF. (C) Quiescent nonconfluent and confluent cells were treated with HGF for the indicated times (minutes), and lysates were immunoblotted with α-pGSK-3β (upper panel) and α-GSK-3β (lower panel). (D) Quiescent nonconfluent cells were pretreated for 20 min with either 10 μM LY294002 or 10 μM Akt IV inhibitor (AktI) and then treated in the presence or absence of HGF for 10 min, and cell lysates were immunoblotted with α-pGSK-3β (upper panel) and α-GSK-3β (lower panel). (E) Quiescent confluent and nonconfluent mIMCD-3 cells were stimulated with HGF for the indicated times and immunoblotted with α-pβ-catenin (upper panel) and α-β-catenin (lower panel). (F) Densitometric quantification of four independent experiments was performed as described for panel E.  , P < 0.01 versus nonconfluent cells in the absence of HGF;
, P < 0.01 versus nonconfluent cells in the absence of HGF; 
 , P < 0.01 versus nonconfluent cells in the presence of HGF for 360 min.
, P < 0.01 versus nonconfluent cells in the presence of HGF for 360 min.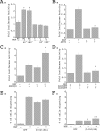
 , P < 0.01 versus nonconfluent cells at time zero and versus confluent cells in the presence of HGF for 60 or 180 min. (B) Cells were transfected as described for panel A and plated under nonconfluent conditions in the presence or absence of Akt IV inhibitor (AKTI) (10 μM) for 1 hour, followed by stimulation in the presence or absence of HGF (40 ng/ml) for 1 hour, and luciferase values were determined and normalized to transfection efficiency. Four independent experiments were performed in triplicate wells.
, P < 0.01 versus nonconfluent cells at time zero and versus confluent cells in the presence of HGF for 60 or 180 min. (B) Cells were transfected as described for panel A and plated under nonconfluent conditions in the presence or absence of Akt IV inhibitor (AKTI) (10 μM) for 1 hour, followed by stimulation in the presence or absence of HGF (40 ng/ml) for 1 hour, and luciferase values were determined and normalized to transfection efficiency. Four independent experiments were performed in triplicate wells.  , P < 0.01 versus HGF stimulated in the absence of the Akt inhibitor. (C) Cells were transfected as described for panel A and plated under nonconfluent conditions in the presence or absence of GSK-3β IX inhibitor (GSK3βI) (1 μM) for 1 hour, followed by stimulation in the presence or absence of HGF (40 ng/ml) for 1 hour, and luciferase values were determined and normalized to transfection efficiency. Three independent experiments were performed in triplicate wells.
, P < 0.01 versus HGF stimulated in the absence of the Akt inhibitor. (C) Cells were transfected as described for panel A and plated under nonconfluent conditions in the presence or absence of GSK-3β IX inhibitor (GSK3βI) (1 μM) for 1 hour, followed by stimulation in the presence or absence of HGF (40 ng/ml) for 1 hour, and luciferase values were determined and normalized to transfection efficiency. Three independent experiments were performed in triplicate wells.  , P < 0.05 versus HGF stimulated in the absence of the GSK-3β inhibitor. (D) Cells were transiently transfected with TOPFLASH and either p-CGN-GSK-3βδ9 or p-CGN-GSK-3βWT, followed by plating under nonconfluent conditions and stimulation in the presence or absence of HGF for 1 hour. Luciferase values were determined and normalized to transfection efficiency. Four independent experiments were performed in triplicate wells.
, P < 0.05 versus HGF stimulated in the absence of the GSK-3β inhibitor. (D) Cells were transiently transfected with TOPFLASH and either p-CGN-GSK-3βδ9 or p-CGN-GSK-3βWT, followed by plating under nonconfluent conditions and stimulation in the presence or absence of HGF for 1 hour. Luciferase values were determined and normalized to transfection efficiency. Four independent experiments were performed in triplicate wells.  , P < 0.01 versus HGF stimulated in the presence of wild-type (WT) GSK-3β. (E) mIMCD-3 cells were transiently transfected with β-catenin (SA) [β-Cat (SA)] and eGFP (GFP) or eGFP alone and plated under nonconfluent conditions in the presence or absence of stimulation with HGF. The percentage of migrating GFP-positive cells was quantitated. Five independent experiments were performed. (F) Cells treated as described for panel E were plated under confluent conditions in the presence or absence of HGF, and the percentage of migrating GFP-positive cells was quantitated. Five independent experiments were performed.
, P < 0.01 versus HGF stimulated in the presence of wild-type (WT) GSK-3β. (E) mIMCD-3 cells were transiently transfected with β-catenin (SA) [β-Cat (SA)] and eGFP (GFP) or eGFP alone and plated under nonconfluent conditions in the presence or absence of stimulation with HGF. The percentage of migrating GFP-positive cells was quantitated. Five independent experiments were performed. (F) Cells treated as described for panel E were plated under confluent conditions in the presence or absence of HGF, and the percentage of migrating GFP-positive cells was quantitated. Five independent experiments were performed.  , P < 0.01 versus confluent cells expressing eGFP alone in the absence of HGF;
, P < 0.01 versus confluent cells expressing eGFP alone in the absence of HGF; 
 , P < 0.01 versus confluent cells expressing eGFP alone in the presence of HGF.
, P < 0.01 versus confluent cells expressing eGFP alone in the presence of HGF.References
-
- Birchmeier, C., W. Birchmeier, E. Gherardi, and G. F. Vande Woude. 2003. Met, metastasis, motility and more. Nat. Rev. Mol. Cell Biol. 4:915-925. - PubMed
-
- Bonventre, J. V., and A. Zuk. 2004. Ischemic acute renal failure: an inflammatory disease? Kidney Int. 66:480-485. - PubMed
-
- Brabletz, T., A. Jung, and T. Kirchner. 2002. Beta-catenin and the morphogenesis of colorectal cancer. Virchows Arch. 441:1-11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
