Perk-dependent translational regulation promotes tumor cell adaptation and angiogenesis in response to hypoxic stress
- PMID: 17030613
- PMCID: PMC1698539
- DOI: 10.1128/MCB.01145-06
Perk-dependent translational regulation promotes tumor cell adaptation and angiogenesis in response to hypoxic stress
Abstract
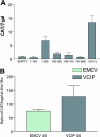
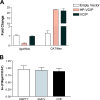
It has been well established that the tumor microenvironment can promote tumor cell adaptation and survival. However, the mechanisms that influence malignant progression have not been clearly elucidated. We have previously demonstrated that cells cultured under hypoxic/anoxic conditions and transformed cells in hypoxic areas of tumors activate a translational control program known as the integrated stress response (ISR). Here, we show that tumors derived from K-Ras-transformed Perk(-/-) mouse embryonic fibroblasts (MEFs) are smaller and exhibit less angiogenesis than tumors with an intact ISR. Furthermore, Perk promotes a tumor microenvironment that favors the formation of functional microvessels. These observations were corroborated by a microarray analysis of polysome-bound RNA in aerobic and hypoxic Perk(+/+) and Perk(-/-) MEFs. This analysis revealed that a subset of proangiogenic transcripts is preferentially translated in a Perk-dependent manner; these transcripts include VCIP, an adhesion molecule that promotes cellular adhesion, integrin binding, and capillary morphogenesis. Taken with the concomitant Perk-dependent translational induction of additional proangiogenic genes identified by our microarray analysis, this study suggests that Perk plays a role in tumor cell adaptation to hypoxic stress by regulating the translation of angiogenic factors necessary for the development of functional microvessels and further supports the contention that the Perk pathway could be an attractive target for novel antitumor modalities.
Figures







References
-
- Ala-aho, R., and V. M. Kahari. 2005. Collagenases in cancer. Biochimie 87:273-286. - PubMed
-
- Ameri, K., E. M. Hammond, C. Culmsee, M. Raida, D. M. Katschinski, R. H. Wenger, E. Wagner, R. J. Davis, T. Hai, N. Denko, and A. L. Harris. 17 July 2006. Induction of activating transcription factor 3 by anoxia is independent of p53 and the hypoxic HIF signalling pathway. Oncogene [Epub ahead of print.] doi:10.1038/sj.onc.1209781. - DOI - PubMed
-
- Ameri, K., C. E. Lewis, M. Raida, H. Sowter, T. Hai, and A. L. Harris. 2004. Anoxic induction of ATF-4 through HIF-1-independent pathways of protein stabilization in human cancer cells. Blood 103:1876-1882. - PubMed
-
- Arsham, A. M., J. J. Howell, and M. C. Simon. 2003. A novel hypoxia-inducible factor-independent hypoxic response regulating mammalian target of rapamycin and its targets. J. Biol. Chem. 278:29655-29660. - PubMed
-
- Bernstein, J., O. Sella, S. Y. Le, and O. Elroy-Stein. 1997. PDGF2/c-sis mRNA leader contains a differentiation-linked internal ribosomal entry site (D-IRES). J. Biol. Chem. 272:9356-9362. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
