The DEK nuclear autoantigen is a secreted chemotactic factor
- PMID: 17030615
- PMCID: PMC1698538
- DOI: 10.1128/MCB.01030-06
The DEK nuclear autoantigen is a secreted chemotactic factor
Abstract
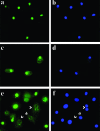
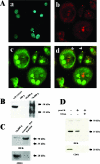
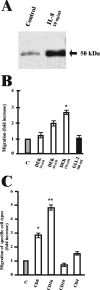
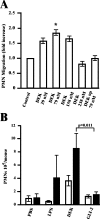
The nuclear DNA-binding protein DEK is an autoantigen that has been implicated in the regulation of transcription, chromatin architecture, and mRNA processing. We demonstrate here that DEK is actively secreted by macrophages and is also found in synovial fluid samples from patients with juvenile arthritis. Secretion of DEK is modulated by casein kinase 2, stimulated by interleukin-8, and inhibited by dexamethasone and cyclosporine A, consistent with a role as a proinflammatory molecule. DEK is secreted in both a free form and in exosomes, vesicular structures in which transcription-modulating factors such as DEK have not previously been found. Furthermore, DEK functions as a chemotactic factor, attracting neutrophils, CD8+ T lymphocytes, and natural killer cells. Therefore, the DEK autoantigen, previously described as a strictly nuclear protein, is secreted and can act as an extracellular chemoattractant, suggesting a direct role for DEK in inflammation.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
- AR48267/AR/NIAMS NIH HHS/United States
- 5 P30 AR48310-02/AR/NIAMS NIH HHS/United States
- M01-RR00042/RR/NCRR NIH HHS/United States
- T32CA88784-03/CA/NCI NIH HHS/United States
- AI40987/AI/NIAID NIH HHS/United States
- R01 AR048267/AR/NIAMS NIH HHS/United States
- T32 GM007863/GM/NIGMS NIH HHS/United States
- R03 AR049907/AR/NIAMS NIH HHS/United States
- AR49907/AR/NIAMS NIH HHS/United States
- T32 GM07863/GM/NIGMS NIH HHS/United States
- P30 AR048310/AR/NIAMS NIH HHS/United States
- M01 RR000042/RR/NCRR NIH HHS/United States
- HL58694/HL/NHLBI NIH HHS/United States
- T32 CA088784/CA/NCI NIH HHS/United States
- R01 AI040987/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
