Neuromedin U receptor 2-deficient mice display differential responses in sensory perception, stress, and feeding
- PMID: 17030627
- PMCID: PMC1698522
- DOI: 10.1128/MCB.01148-06
Neuromedin U receptor 2-deficient mice display differential responses in sensory perception, stress, and feeding
Abstract
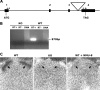
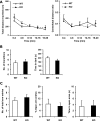
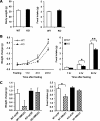
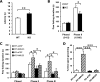
Neuromedin U (NMU) is a highly conserved neuropeptide with a variety of physiological functions mediated by two receptors, peripheral NMUR1 and central nervous system NMUR2. Here we report the generation and phenotypic characterization of mice deficient in the central nervous system receptor NMUR2. We show that behavioral effects, such as suppression of food intake, enhanced pain response, and excessive grooming induced by intracerebroventricular NMU administration were abolished in the NMUR2 knockout (KO) mice, establishing a causal role for NMUR2 in mediating NMU's central effects on these behaviors. In contrast to the NMU peptide-deficient mice, NMUR2 KO mice appeared normal with regard to stress, anxiety, body weight regulation, and food consumption. However, the NMUR2 KO mice showed reduced pain sensitivity in both the hot plate and formalin tests. Furthermore, facilitated excitatory synaptic transmission in spinal dorsal horn neurons, a mechanism by which NMU stimulates pain, did not occur in NMUR2 KO mice. These results provide significant insights into a functional dissection of the differential contribution of peripherally or centrally acting NMU system. They suggest that NMUR2 plays a more significant role in central pain processing than other brain functions including stress/anxiety and regulation of feeding.
Figures


References
-
- Amit, Z., and Z. H. Galina. 1986. Stress-induced analgesia: adaptive pain suppression. Physiol. Rev. 66:1091-1120. - PubMed
-
- Arancio, O., E. R. Kandel, and R. D. Hawkins. 1995. Activity-dependent long-term enhancement of transmitter release by presynaptic 3′,5′-cyclic GMP in cultured hippocampal neurons. Nature 376:74-80. - PubMed
-
- Brighton, P. J., P. G. Szekeres, and G. B. Willars. 2004. Neuromedin U and its receptors: structure, function, and physiological roles. Pharmacol. Rev. 56:231-248. - PubMed
-
- Cao, C. Q., X. H. Yu, A. Dray, A. Filosa, and M. N. Perkins. 2003. A pro-nociceptive role of neuromedin U in adult mice. Pain 104:609-616. - PubMed
-
- Craig, A. D. 1996. An ascending general homeostatic afferent pathway originating in lamina I. Prog. Brain Res. 107:225-242. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
