Angiopoietin-2 functions as an autocrine protective factor in stressed endothelial cells
- PMID: 17030814
- PMCID: PMC1592534
- DOI: 10.1073/pnas.0607538103
Angiopoietin-2 functions as an autocrine protective factor in stressed endothelial cells
Abstract
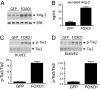
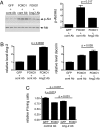
Angiopoietin (Ang)-2, a context-dependent agonist/antagonist for the vascular-specific Tie2 receptor, is highly expressed by endothelial cells at sites of normal and pathologic angiogenesis. One prevailing model suggests that in these settings, Ang-2 acts as an autocrine Tie2 blocker, inhibiting the stabilizing influence of the Tie2 activator Ang-1, thereby promoting vascular remodeling. However, the effects of endogenous Ang-2 on cells that are actively producing it have not been studied in detail. Here, we demonstrate that Ang-2 expression is rapidly induced in endothelial cells by the transcription factor FOXO1 after inhibition of the phosphatidylinositol 3-kinase/Akt pathway. We employ RNAi and blocking antibodies to show that in this setting, Ang-2 unexpectedly functions as a Tie2 agonist, bolstering Akt activity so as to provide negative feedback on FOXO1-regulated transcription and apoptosis. In addition, we show that Ang-2, like Ang-1, activates Tie2/Akt signaling in vivo, thereby inhibiting the expression of FOXO1 target genes. Consistent with a role for Ang-2 as a Tie2 activator, we demonstrate that Ang-2 inhibits vascular leak. Our data suggests a model in which Ang-2 expression is induced in stressed endothelial cells, where it acts as an autocrine Tie2 agonist and protective factor.
Conflict of interest statement
Conflict of interest statement: All of the authors except one (D.M.M.) are employees of, and own stock or stock options in, Regeneron Pharmaceuticals.
Figures


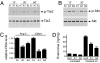
References
-
- Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, Gridley T, Wolburg H, Risau W, Qin Y. Nature. 1995;376:70–74. - PubMed
-
- Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC, Yancopoulos GD. Cell. 1996;87:1161–1169. - PubMed
-
- Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Cell. 1996;87:1171–1180. - PubMed
-
- Davis S, Papadopoulos N, Aldrich TH, Maisonpierre PC, Huang T, Kovac L, Xu A, Leidich R, Radziejewska E, Rafique A, et al. Nat Struct Biol. 2003;10:38–44. - PubMed
-
- Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, et al. Science. 1997;277:55–60. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

