Strict host-symbiont cospeciation and reductive genome evolution in insect gut bacteria
- PMID: 17032065
- PMCID: PMC1592312
- DOI: 10.1371/journal.pbio.0040337
Strict host-symbiont cospeciation and reductive genome evolution in insect gut bacteria
Abstract
Host-symbiont cospeciation and reductive genome evolution have been identified in obligate endocellular insect symbionts, but no such example has been identified from extracellular ones. Here we first report such a case in stinkbugs of the family Plataspidae, wherein a specific gut bacterium is vertically transmitted via "symbiont capsule." In all of the plataspid species, females produced symbiont capsules upon oviposition and their gut exhibited specialized traits for capsule production. Phylogenetic analysis showed that the plataspid symbionts constituted a distinct group in the gamma-Proteobacteria, whose sister group was the aphid obligate endocellular symbionts Buchnera. Removal of the symbionts resulted in retarded growth, mortality, and sterility of the insects. The host phylogeny perfectly agreed with the symbiont phylogeny, indicating strict host-symbiont cospeciation despite the extracellular association. The symbionts exhibited AT-biased nucleotide composition, accelerated molecular evolution, and reduced genome size, as has been observed in obligate endocellular insect symbionts. These findings suggest that not the endocellular conditions themselves but the population genetic attributes of the vertically transmitted symbionts are probably responsible for the peculiar genetic traits of these insect symbionts. We proposed the designation "Candidatus Ishikawaella capsulata" for the plataspid symbionts. The plataspid stinkbugs, wherein the host-symbiont associations can be easily manipulated, provide a novel system that enables experimental approaches to previously untouched aspects of the insect-microbe mutualism. Furthermore, comparative analyses of the sister groups, the endocellular Buchnera and the extracellular Ishikawaella, would lead to insights into how the different symbiotic lifestyles have affected their genomic evolution.
Conflict of interest statement
Competing interests. The authors have declared that no competing interests exist.
Figures
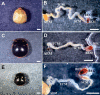
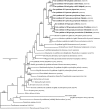

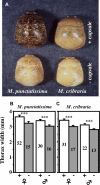


Comment in
-
Gut bacteria cospeciating with insects.PLoS Biol. 2006 Oct;4(10):e357. doi: 10.1371/journal.pbio.0040357. Epub 2006 Oct 10. PLoS Biol. 2006. PMID: 20076479 Free PMC article. No abstract available.
References
-
- Baumann P, Moran NA, Baumann L. Bacteriocyte-associated endosymbionts of insects. In: Dworkin M, editor. The prokaryotes. New York: Springer; 2000. pp. 1–55.
-
- Bourtzis K, Miller T. Insect symbiosis. Boca Raton: CRC Press; 2003. 347
-
- Buchner P. Endosymbiosis of animals with plant microorganisms. New York: Interscience; 1965. 909
-
- Douglas AE. Nutritional interactions in insect-microbial symbioses: Aphids and their symbiotic bacteria Buchnera . Ann Rev Entomol. 1998;43:17–37. - PubMed
-
- Shigenobu S, Watanabe H, Hattori M, Sakai Y, Ishikawa H. Genome sequence of the endocellular bacterial symbiont of aphids Buchnera sp. APS. Nature. 2000;407:81–86. - PubMed
Publication types
MeSH terms
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

