The impact of altered p53 dosage on hematopoietic stem cell dynamics during aging
- PMID: 17032926
- PMCID: PMC1794064
- DOI: 10.1182/blood-2006-03-010413
The impact of altered p53 dosage on hematopoietic stem cell dynamics during aging
Abstract
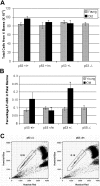
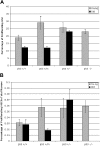
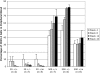
A temporal decline in tissue stem cell functionality may be a key component of mammalian aging. The tumor suppressor p53 has recently been implicated as a potential regulator of aging. We examined age-associated hematopoietic stem cell (HSC) dynamics in mice with varying p53 activities. Reduced p53 activity in p53+/- mice was associated with higher numbers of proliferating hematopoietic stem and progenitor cells in old age compared with aged wild-type (p53+/+) mice. We also assessed HSC dynamics in a p53 mutant mouse model (p53+/m) with higher apparent p53 activity than wild-type mice. The p53 hypermorphic (p53+/m) mice display phenotypes of premature aging. Many aged p53+/m organs exhibit reduced cellularity and atrophy, suggesting defects in stem-cell regenerative capacity. HSC numbers from old p53+/m mice fail to increase with age, unlike those of their p53+/+ and p53+/- counterparts. Moreover, transplantation of 500 HSCs from old p53+/m mice into lethally irradiated recipients resulted in reduced engraftment compared with old wild-type p53+/+ and p53+/- HSCs. Thus, alteration of p53 activity affects stem-cell numbers, proliferation potential, and hematopoiesis in older organisms, supporting a model in which aging is caused in part by a decline in tissue stem cell regenerative function.
Figures
References
-
- Weissman IL. Stem cells: units of development, units of regeneration, and units in evolution. Cell. 2000;100:157–168. - PubMed
-
- Van Zant G, Liang Y. The role of stem cells in aging. Exp Hematol. 2003;31:659–672. - PubMed
-
- Chen J. Senescence and functional failure in hematopoietic stem cells. Exp Hematol. 2004;32:1025–1032. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

