Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism
- PMID: 17033621
- PMCID: PMC1839871
- DOI: 10.1038/ng1905
Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism
Abstract
The osteocyte, a terminally differentiated cell comprising 90%-95% of all bone cells, may have multiple functions, including acting as a mechanosensor in bone (re)modeling. Dentin matrix protein 1 (encoded by DMP1) is highly expressed in osteocytes and, when deleted in mice, results in a hypomineralized bone phenotype. We investigated the potential for this gene not only to direct skeletal mineralization but also to regulate phosphate (P(i)) homeostasis. Both Dmp1-null mice and individuals with a newly identified disorder, autosomal recessive hypophosphatemic rickets, manifest rickets and osteomalacia with isolated renal phosphate-wasting associated with elevated fibroblast growth factor 23 (FGF23) levels and normocalciuria. Mutational analyses showed that autosomal recessive hypophosphatemic rickets family carried a mutation affecting the DMP1 start codon, and a second family carried a 7-bp deletion disrupting the highly conserved DMP1 C terminus. Mechanistic studies using Dmp1-null mice demonstrated that absence of DMP1 results in defective osteocyte maturation and increased FGF23 expression, leading to pathological changes in bone mineralization. Our findings suggest a bone-renal axis that is central to guiding proper mineral metabolism.
Figures

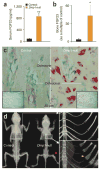


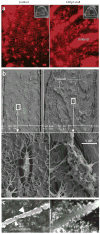

Comment in
-
Bone talk.Nat Genet. 2006 Nov;38(11):1230-1. doi: 10.1038/ng1106-1230. Nat Genet. 2006. PMID: 17072297 No abstract available.
References
-
- Frost HM. In vivo osteocyte death. J Bone Joint Surg Am. 1960;42A:138–143. - PubMed
-
- Palumbo C, Palazzini S, Zaffe D, Marotti G. Osteocyte differentiation in the tibia of newborn rabbit: an ultrastructural study of the formation of cytoplasmic processes. Acta Anat (Basel) 1990;137:350–358. - PubMed
-
- Pead MJ, Lanyon LE. Indomethacin modulation of load-related stimulation of new bone formation in vivo. Calcif Tissue Int. 1989;45:34–40. - PubMed
-
- Toyosawa S, et al. Dentin matrix protein 1 is predominantly expressed in chicken and rat osteocytes but not in osteoblasts. J Bone Miner Res. 2001;16:2017–2026. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R56 AR045955/AR/NIAMS NIH HHS/United States
- R56 AR027032/AR/NIAMS NIH HHS/United States
- DK063934/DK/NIDDK NIH HHS/United States
- AR027032/AR/NIAMS NIH HHS/United States
- P01 AR046798/AR/NIAMS NIH HHS/United States
- AR-45955/AR/NIAMS NIH HHS/United States
- AR051587/AR/NIAMS NIH HHS/United States
- R29 DE013480/DE/NIDCR NIH HHS/United States
- R01 AR045955/AR/NIAMS NIH HHS/United States
- AR046798/AR/NIAMS NIH HHS/United States
- DE13480/DE/NIDCR NIH HHS/United States
- R01 DK063934/DK/NIDDK NIH HHS/United States
- R01 AR027032/AR/NIAMS NIH HHS/United States
- R01 AR051587/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

