Mutations in the tight-junction gene claudin 19 (CLDN19) are associated with renal magnesium wasting, renal failure, and severe ocular involvement
- PMID: 17033971
- PMCID: PMC1698561
- DOI: 10.1086/508617
Mutations in the tight-junction gene claudin 19 (CLDN19) are associated with renal magnesium wasting, renal failure, and severe ocular involvement
Abstract
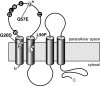
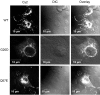
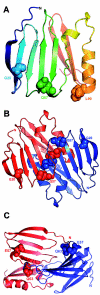
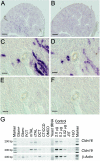
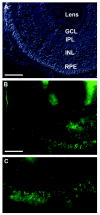
Claudins are major components of tight junctions and contribute to the epithelial-barrier function by restricting free diffusion of solutes through the paracellular pathway. We have mapped a new locus for recessive renal magnesium loss on chromosome 1p34.2 and have identified mutations in CLDN19, a member of the claudin multigene family, in patients affected by hypomagnesemia, renal failure, and severe ocular abnormalities. CLDN19 encodes the tight-junction protein claudin-19, and we demonstrate high expression of CLDN19 in renal tubules and the retina. The identified mutations interfere severely with either cell-membrane trafficking or the assembly of the claudin-19 protein. The identification of CLDN19 mutations in patients with chronic renal failure and severe visual impairment supports the fundamental role of claudin-19 for normal renal tubular function and undisturbed organization and development of the retina.
Figures



References
Web Resources
-
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for Cldn16 [accession number NM_053241] and Cldn19 [accession number BC115827])
-
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for FHHNC) - PubMed
References
-
- Colegio OR, Van Itallie CM, McCrea HJ, Rahner C, Anderson JM (2002) Claudins create charge-selective channels in the paracellular pathway between epithelial cells. Am J Physiol Cell Physiol 283:C142–C147 - PubMed
-
- Van Itallie CM, Fanning AS, Anderson JM (2003) Reversal of charge selectivity in cation or anion-selective epithelial lines by expression of different claudins. Am J Physiol Renal Physiol 285:F1078–F1084 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

