Pyrrolidine carboxamides as a novel class of inhibitors of enoyl acyl carrier protein reductase from Mycobacterium tuberculosis
- PMID: 17034137
- PMCID: PMC2517584
- DOI: 10.1021/jm060715y
Pyrrolidine carboxamides as a novel class of inhibitors of enoyl acyl carrier protein reductase from Mycobacterium tuberculosis
Abstract
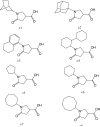
In view of the worldwide spread of multidrug resistance of Mycobacterium tuberculosis, there is an urgent need to discover antituberculosis agent with novel structures. InhA, the enoyl acyl carrier protein reductase (ENR) from M. tuberculosis, is one of the key enzymes involved in the mycobacterial fatty acid elongation cycle and has been validated as an effective antimicrobial target. We report here the discovery, through high-throughput screening, of a series of pyrrolidine carboxamides as a novel class of potent InhA inhibitors. Crystal structures of InhA complexed with three inhibitors have been used to elucidate the inhibitor binding mode. The potency of the lead compound was improved over 160-fold by subsequent optimization through iterative microtiter library synthesis followed by in situ activity screening without purification. Resolution of racemic mixtures of several inhibitors indicate that only one enantiomer is active as an inhibitor of InhA.
Figures
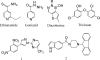
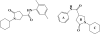


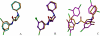

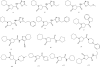



References
-
- Tuberculosis, NIAID Fact Sheet http://www.niaid.nih.gov/factsheets/tb.htm.
-
- Global Alliance for TB Drug Development ( www.tballiance.org)
-
- WHO fact sheet http://www.who.int/mediacentre/factsheets/who104/en/print.html.
-
- Campbell JW, Cronan JE., Jr. Bacterial fatty acid biosynthesis: targets for antibacterial drug discovery. Annu. Rev. Microbiol. 2001;55:305–332. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

