Understanding and meeting the needs of those using growth hormone injection devices
- PMID: 17034628
- PMCID: PMC1618831
- DOI: 10.1186/1472-6823-6-5
Understanding and meeting the needs of those using growth hormone injection devices
Abstract
Background: Recombinant human growth hormone (r-hGH) is used to treat: growth hormone deficiency in children and adults; children born small for gestational age; Turner's syndrome; and chronic renal failure. r-hGH is administered by daily subcutaneous injection and may be given using a number of different administration devices. The aim of this survey was, firstly, to identify which attributes of an r-hGH administration device are considered most important to physicians, teenage patients, parents of young children requiring GH and nurses who have experience of r-hGH administration, and, secondly, to determine how they rate existing devices in each of these key attributes.
Methods: The opinions of 67 individuals with experience in r-hGH administration were captured in discussion sessions. Parents, physicians and nurses were asked to rate 19 device attributes by completing a questionnaire, and to rank four different r-hGH administration devices (including a conceptual electronic device) in order of preference.
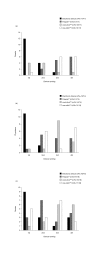
Results: Reliability, ease of use, lack of pain during injection, safety in use, storage, and number of steps in preparation before use, during use and after were considered to be the five most desirable attributes of an r-hGH administration device. An electronic device was preferred to an automatic, multi-dose injection device, a needle-free injection device or a manual, ready-to-use, disposable injection device.
Conclusion: In the opinion of physicians, nurses and parents using r-hGH injection devices, an ideal device must combine reliability with simplicity, while delivering treatment with minimal pain. An electronic device, which combines many of the most useful features of existing devices with novel functions, was the preferred option for r-hGH administration.
Figures


References
-
- Preece MA. Diagnosis and treatment of children with growth hormone deficiency. Clinical Endocrinology and Metabolism. 1982;11:1–24. - PubMed
-
- McGauley G. The psychological consequences and quality of life in adults with growth hormone deficiency. Growth Hormone and IGF Research. 2000;10 Suppl B:S63–S68. - PubMed
-
- Raben MS. Treatment of a pituitary dwarf with human growth hormone. Journal of Clinical Endocrinology and Metabolism. 1958;18:901–903. - PubMed
-
- Verhelst J, Abs R, Vandeweghe M, Mockel J, Legros JJ, Copinschi G, Mahler C, Velkeniers B, Vanhaelst L, Van Aelst A, De Rijdt D, Stevenaert A, Beckers A. Two years of replacement therapy in adults with growth hormone deficiency. Clinical Endocrinology. 1997;47:485–494. doi: 10.1046/j.1365-2265.1997.3041112.x. - DOI - PubMed
LinkOut - more resources
Full Text Sources

