Modeling early cortical serotonergic deficits in autism
- PMID: 17034875
- PMCID: PMC2570481
- DOI: 10.1016/j.bbr.2006.08.026
Modeling early cortical serotonergic deficits in autism
Abstract
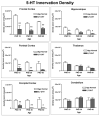
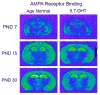
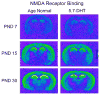
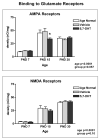
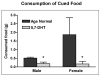
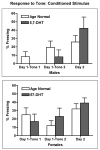
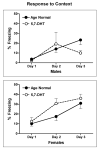
Autism is a developmental brain disorder characterized by deficits in social interaction, language and behavior. Brain imaging studies demonstrate increased cerebral cortical volumes and micro- and macro-scopic neuroanatomic changes in children with this disorder. Alterations in forebrain serotonergic function may underlie the neuroanatomic and behavioral features of autism. Serotonin is involved in neuronal growth and plasticity and these actions are likely mediated via serotonergic and glutamatergic receptors. Few animal models of autism have been described that replicate both etiology and pathophysiology. We report here on a selective serotonin (5-HT) depletion model of this disorder in neonatal mice that mimics neurochemical and structural changes in cortex and, in addition, displays a behavioral phenotype consistent with autism. Newborn male and female mice were depleted of forebrain 5-HT with injections of the serotonergic neurotoxin, 5,7-dihydroxytryptamine (5,7-DHT), into the bilateral medial forebrain bundle (mfb). Behavioral testing of these animals as adults revealed alterations in social, sensory and stereotypic behaviors. Lesioned mice showed significantly increased cortical width. Serotonin immunocytochemistry showed a dramatic long-lasting depletion of 5-HT containing fibers in cerebral cortex until postnatal day (PND) 60. Autoradiographic binding to high affinity 5-HT transporters was significantly but transiently reduced in cerebral cortex of 5,7-DHT-depleted mice. AMPA glutamate receptor binding was decreased at PND 15. We hypothesize that increased cerebral cortical volume and sensorimotor, cognitive and social deficits observed in both 5-HT-depleted animals and in individuals with autism, may be the result of deficiencies in timely axonal pruning to key cerebral cortical areas.
Figures


References
-
- Akshoomoff N, Lord C, Lincoln AJ, Courchesne RY, Carper RA, Townsend J, Courchesne E. Outcome classification of preschool children with autism spectrum disorders using MRI brain measures. J Am Acad Child Adolesc Psychiatry. 2004;43:349–357. - PubMed
-
- Anderson GM, Horne WC, Chatterjee D, Cohen DJ. The hyperserotonemia of autism. Ann N Y Acad Sci. 1990;600:331–340. discussion 341–332. - PubMed
-
- Aylward EH, Minshew NJ, Field K, Sparks BF, Singh N. Effects of age on brain volume and head circumference in autism. Neurology. 2002;59:175–183. - PubMed
-
- Bailey A, Luthert P, Dean A, Harding B, Janota I, Montgomery M, Rutter M, Lantos P. A clinicopathological study of autism. Brain. 1998;121 ( Pt 5):889–905. - PubMed
-
- Baranek GT. Autism during infancy: a retrospective video analysis of sensory-motor and social behaviors at 9–12 months of age. J Autism Dev Disord. 1999;29:213–224. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

