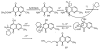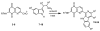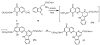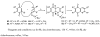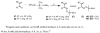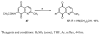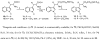Synthesis and evaluation of antitumor activity of novel N-acyllavendamycin analogues and quinoline-5,8-diones
- PMID: 17035024
- PMCID: PMC1900071
- DOI: 10.1016/j.bmc.2006.09.039
Synthesis and evaluation of antitumor activity of novel N-acyllavendamycin analogues and quinoline-5,8-diones
Abstract
A series of 7-N-acyllavendamycins with zero, one or two substituents at the C-2', C-3', and C-11' were synthesized through short and efficient methods. Pictet-Spengler condensation of 7-N-acylamino-2-formylquinoline-5,8-diones with tryptamine or tryptophans produced the desired lavendamycins. Screening data on a panel of three ras oncogene-transformed cell lines and the non-transformed parent cell line showed that a significant number of these analogues are potent antitumor agents and appear to be particularly active against K-ras transformed cells. Compared with the corresponding quinolinediones, these novel lavendamycins are much more inhibitory toward the transformed cells indicating that the beta-carboline moiety of the lavendamycin analogues plays an important role in its potency and selective toxicity.
Figures
Similar articles
-
Synthesis, metabolism and in vitro cytotoxicity studies on novel lavendamycin antitumor agents.Bioorg Med Chem. 2010 Mar 1;18(5):1899-909. doi: 10.1016/j.bmc.2010.01.037. Epub 2010 Jan 25. Bioorg Med Chem. 2010. PMID: 20149966 Free PMC article.
-
Novel lavendamycin analogues as potent HIV-reverse transcriptase inhibitors: synthesis and evaluation of anti-reverse transcriptase activity of amide and ester analogues of lavendamycin.J Med Chem. 2003 Dec 18;46(26):5773-80. doi: 10.1021/jm0304414. J Med Chem. 2003. PMID: 14667230
-
Novel lavendamycin analogues as antitumor agents: synthesis, in vitro cytotoxicity, structure-metabolism, and computational molecular modeling studies with NAD(P)H:quinone oxidoreductase 1.J Med Chem. 2005 Dec 1;48(24):7733-49. doi: 10.1021/jm050758z. J Med Chem. 2005. PMID: 16302813
-
A Review on the Synthesis and Anti-cancer Activity of 2-substituted Quinolines.Anticancer Agents Med Chem. 2015;15(5):631-46. doi: 10.2174/1871520615666141216125446. Anticancer Agents Med Chem. 2015. PMID: 25511516 Review.
-
5,8-Quinolinedione Scaffold as a Promising Moiety of Bioactive Agents.Molecules. 2019 Nov 14;24(22):4115. doi: 10.3390/molecules24224115. Molecules. 2019. PMID: 31739496 Free PMC article. Review.
Cited by
-
Quinoline and naphthalene derivatives from Saccharopolyspora sp. YIM M13568.J Antibiot (Tokyo). 2017 Mar;70(3):320-322. doi: 10.1038/ja.2016.142. Epub 2016 Nov 30. J Antibiot (Tokyo). 2017. PMID: 27899791 No abstract available.
-
An efficient synthesis of 4H-pyrano quinolinone derivatives catalysed by a versatile organocatalyst tetra-n-butylammonium fluoride and their pharmacological screening.R Soc Open Sci. 2017 Nov 29;4(11):170764. doi: 10.1098/rsos.170764. eCollection 2017 Nov. R Soc Open Sci. 2017. PMID: 29291069 Free PMC article.
-
Small molecule PZL318: forming fluorescent nanoparticles capable of tracing their interactions with cancer cells and activated platelets, slowing tumor growth and inhibiting thrombosis.Int J Nanomedicine. 2015 Aug 24;10:5273-92. doi: 10.2147/IJN.S88052. eCollection 2015. Int J Nanomedicine. 2015. PMID: 26345234 Free PMC article.
-
New arylated benzo[h]quinolines induce anti-cancer activity by oxidative stress-mediated DNA damage.Sci Rep. 2016 Dec 6;6:38128. doi: 10.1038/srep38128. Sci Rep. 2016. PMID: 27922047 Free PMC article.
-
Synthesis, metabolism and in vitro cytotoxicity studies on novel lavendamycin antitumor agents.Bioorg Med Chem. 2010 Mar 1;18(5):1899-909. doi: 10.1016/j.bmc.2010.01.037. Epub 2010 Jan 25. Bioorg Med Chem. 2010. PMID: 20149966 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous