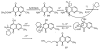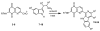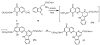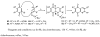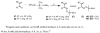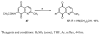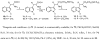Synthesis and evaluation of antitumor activity of novel N-acyllavendamycin analogues and quinoline-5,8-diones
- PMID: 17035024
- PMCID: PMC1900071
- DOI: 10.1016/j.bmc.2006.09.039
Synthesis and evaluation of antitumor activity of novel N-acyllavendamycin analogues and quinoline-5,8-diones
Abstract
A series of 7-N-acyllavendamycins with zero, one or two substituents at the C-2', C-3', and C-11' were synthesized through short and efficient methods. Pictet-Spengler condensation of 7-N-acylamino-2-formylquinoline-5,8-diones with tryptamine or tryptophans produced the desired lavendamycins. Screening data on a panel of three ras oncogene-transformed cell lines and the non-transformed parent cell line showed that a significant number of these analogues are potent antitumor agents and appear to be particularly active against K-ras transformed cells. Compared with the corresponding quinolinediones, these novel lavendamycins are much more inhibitory toward the transformed cells indicating that the beta-carboline moiety of the lavendamycin analogues plays an important role in its potency and selective toxicity.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous