3D pattern of brain atrophy in HIV/AIDS visualized using tensor-based morphometry
- PMID: 17035049
- PMCID: PMC3197835
- DOI: 10.1016/j.neuroimage.2006.08.030
3D pattern of brain atrophy in HIV/AIDS visualized using tensor-based morphometry
Abstract
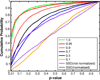
35% of HIV-infected patients have cognitive impairment, but the profile of HIV-induced brain damage is still not well understood. Here we used tensor-based morphometry (TBM) to visualize brain deficits and clinical/anatomical correlations in HIV/AIDS. To perform TBM, we developed a new MRI-based analysis technique that uses fluid image warping, and a new alpha-entropy-based information-theoretic measure of image correspondence, called the Jensen-Rényi divergence (JRD).
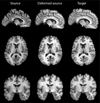
Methods: 3D T1-weighted brain MRIs of 26 AIDS patients (CDC stage C and/or 3 without HIV-associated dementia; 47.2+/-9.8 years; 25M/1F; CD4+ T-cell count: 299.5+/-175.7/microl; log10 plasma viral load: 2.57+/- 1.28 RNA copies/ml) and 14 HIV-seronegative controls (37.6+/-12.2 years; 8M/6F) were fluidly registered by applying forces throughout each deforming image to maximize the JRD between it and a target image (from a control subject). The 3D fluid registration was regularized using the linearized Cauchy-Navier operator. Fine-scale volumetric differences between diagnostic groups were mapped. Regions were identified where brain atrophy correlated with clinical measures.
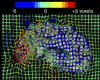
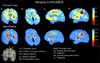
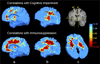
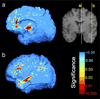
Results: Severe atrophy ( approximately 15-20% deficit) was detected bilaterally in the primary and association sensorimotor areas. Atrophy of these regions, particularly in the white matter, correlated with cognitive impairment (P = 0.033) and CD4+ T-lymphocyte depletion (P = 0.005).
Conclusion: TBM facilitates 3D visualization of AIDS neuropathology in living patients scanned with MRI. Severe atrophy in frontoparietal and striatal areas may underlie early cognitive dysfunction in AIDS patients, and may signal the imminent onset of AIDS dementia complex.
Figures




References
-
- Arsigny V, Fillard P, Pennec X, Ayache N. Fast and simple calculus on tensors in the Log-Euclidean framework. Proceedings of the 8th Int. Conf. on Medical Image Computing and Computer-Assisted Intervention—MICCAI 2005; October 26–29; Palm Springs, CA, USA. 2005. - PubMed
-
- Ashburner J, Friston KJ. Voxel-based morphometry—The methods. NeuroImage. 2000;11(6):805–821. - PubMed
-
- Avants B, Gee JC. Geodesic estimation for large deformation anatomical shape averaging and interpolation. NeuroImage. 2004;23 Suppl. 1:S139–S150. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

