Functional analyses of glycyl-tRNA synthetase mutations suggest a key role for tRNA-charging enzymes in peripheral axons
- PMID: 17035524
- PMCID: PMC6674701
- DOI: 10.1523/JNEUROSCI.1671-06.2006
Functional analyses of glycyl-tRNA synthetase mutations suggest a key role for tRNA-charging enzymes in peripheral axons
Abstract
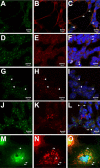
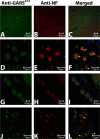
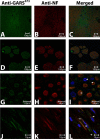
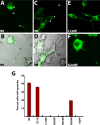
Charcot-Marie-Tooth disease type 2D (CMT2D) and distal spinal muscular atrophy type V (dSMA-V) are axonal neuropathies characterized by a phenotype that is more severe in the upper extremities. We previously implicated mutations in the gene encoding glycyl-tRNA synthetase (GARS) as the cause of CMT2D and dSMA-V. GARS is a member of the family of aminoacyl-tRNA synthetases responsible for charging tRNA with cognate amino acids; GARS ligates glycine to tRNA(Gly). Here, we present functional analyses of disease-associated GARS mutations and show that there are not any significant mutation-associated changes in GARS expression levels; that the majority of identified GARS mutations modeled in yeast severely impair viability; and that, in most cases, mutant GARS protein mislocalizes in neuronal cells. Indeed, four of the five mutations studied show loss-of-function features in at least one assay, suggesting that tRNA-charging deficits play a role in disease pathogenesis. Finally, we detected endogenous GARS-associated granules in the neurite projections of cultured neurons and in the peripheral nerve axons of normal human tissue. These data are particularly important in light of the recent identification of CMT-associated mutations in another tRNA synthetase gene [YARS (tyrosyl-tRNA synthetase gene)]. Together, these findings suggest that tRNA-charging enzymes play a key role in maintaining peripheral axons.
Figures


References
-
- Antonellis A, Ellsworth RE, Sambuughin N, Puls I, Abel A, Lee-Lin SQ, Jordanova A, Kremensky I, Christodoulou K, Middleton LT, Sivakumar K, Ionasescu V, Funalot B, Vance JM, Goldfarb LG, Fischbeck KH, Green ED. Glycyl tRNA synthetase mutations in Charcot–Marie–Tooth disease type 2D and distal spinal muscular atrophy type V. Am J Hum Genet. 2003;72:1293–1299. - PMC - PubMed
-
- Biedler JL, Roffler-Tarlov S, Schachner M, Freedman LS. Multiple neurotransmitter synthesis by human neuroblastoma cell lines and clones. Cancer Res. 1978;38:3751–3757. - PubMed
-
- Boeke JD, LaCroute F, Fink GR. A positive selection for mutants lacking orotidine-5′-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol Gen Genet. 1984;197:345–346. - PubMed
-
- Christodoulou K, Kyriakides T, Hristova AH, Georgiou DM, Kalaydjieva L, Yshpekova B, Ivanova T, Weber JL, Middleton LT. Mapping of a distal form of spinal muscular atrophy with upper limb predominance to chromosome 7p. Hum Mol Genet. 1995;4:1629–1632. - PubMed
-
- Corti O, Hampe C, Koutnikova H, Darios F, Jacquier S, Prigent A, Robinson JC, Pradier L, Ruberg M, Mirande M, Hirsch E, Rooney T, Fournier A, Brice A. The p38 subunit of the aminoacyl-tRNA synthetase complex is a Parkin substrate: linking protein biosynthesis and neurodegeneration. Hum Mol Genet. 2003;12:1427–1437. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
