The transcription factor six1 inhibits neuronal and promotes hair cell fate in the developing zebrafish (Danio rerio) inner ear
- PMID: 17035528
- PMCID: PMC6674689
- DOI: 10.1523/JNEUROSCI.1025-06.2006
The transcription factor six1 inhibits neuronal and promotes hair cell fate in the developing zebrafish (Danio rerio) inner ear
Abstract
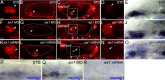
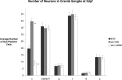
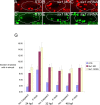
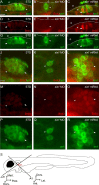
The developmental processes leading to the differentiation of mechanosensory hair cells and statoacoustic ganglion neurons from the early otic epithelium remain unclear. Possible candidates include members of the Pax-Six-Eya-Dach (paired box-sine oculis homeobox-eyes absent-dachshund) gene regulatory network. We cloned zebrafish six1 and studied its function in inner ear development. Gain- and loss-of-function experiments show that six1 has opposing roles in hair cell and neuronal lineages. It promotes hair cell fate and, conversely, inhibits neuronal fate by differentially affecting cell proliferation and cell death in these lineages. By independently targeting hair cells with atoh1a (atonal homolog 1a) knockdown or neurons with neurog1 (neurogenin 1) knockdown, we showed that the remaining cell population, neurons or hair cells, respectively, is still affected by gain or loss of six1 function. six1 interacts with other members of the Pax-Six-Eya-Dach regulatory network, in particular dacha and dachb in the hair cell but not neuronal lineage. Unlike in mouse, six1 does not appear to be dependent on eya1, although it seems to be important for the regulation of eya1 and pax2b expression in the ventral otic epithelium. Furthermore, six1 expression appears to be regulated by pax2b and also by foxi1 (forkhead box I1) as expected for an early inducer of the otic placode. Our results are the first to demonstrate a dual role for a member of the Pax-Six-Eya-Dach regulatory network in inner ear development.
Figures


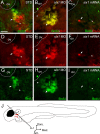
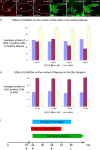
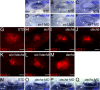

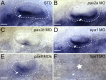
References
-
- Andermann P, Ungos J, Raible DW. Neurogenin1 defines zebrafish cranial sensory ganglia precursors. Dev Biol. 2002;251:45–58. - PubMed
-
- Appel B, Eisen JS. Regulation of neuronal specification in the zebrafish spinal cord by Delta function. Development. 1998;125:371–380. - PubMed
-
- Baker CV, Bronner-Fraser M. Vertebrate cranial placodes. I. Embryonic induction. Dev Biol. 2001;232:1–61. - PubMed
-
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–1841. - PubMed
-
- Bessarab DA, Chong SW, Korzh V. Expression of zebrafish six1 during sensory organ development and myogenesis. Dev Dyn. 2004;230:781–786. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
