Acute impairment of mitochondrial trafficking by beta-amyloid peptides in hippocampal neurons
- PMID: 17035532
- PMCID: PMC6674697
- DOI: 10.1523/JNEUROSCI.3231-06.2006
Acute impairment of mitochondrial trafficking by beta-amyloid peptides in hippocampal neurons
Abstract
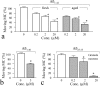
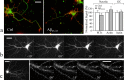
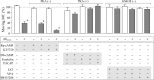
Defects in axonal transport are often associated with a wide variety of neurological diseases including Alzheimer's disease (AD). Beta-amyloid (Abeta) is a major component of neuritic plaques associated with pathological conditions of AD brains. Here, we report that a brief exposure of cultured hippocampal neurons to Abeta molecules resulted in rapid and severe impairment of mitochondrial transport without inducing apparent cell death and significant morphological changes. Such acute inhibition of mitochondrial transport was not associated with a disruption of mitochondria potential nor involved aberrant cytoskeletal changes. Abeta also did not elicit significant Ca2+ signaling to affect mitochondrial trafficking. However, stimulation of protein kinase A (PKA) by forskolin, cAMP analogs, or neuropeptides effectively alleviated the impairment. We also show that Abeta inhibited mitochondrial transport by acting through glycogen synthase kinase 3beta (GSK3beta). Given that mitochondria are crucial organelles for many cellular functions and survival, our findings thus identify an important acute action of Abeta molecules on nerve cells that could potentially contribute to various abnormalities of neuronal functions under AD conditions. Manipulation of GSK3beta and PKA activities may represent a key approach for preventing and alleviating Abeta cytotoxicity and AD pathological conditions.
Figures




References
-
- Abramov AY, Canevari L, Duchen MR. Calcium signals induced by amyloid beta peptide and their consequences in neurons and astrocytes in culture. Biochim Biophys Acta. 2004b;1742:81–87. - PubMed
-
- Bacci A, Verderio C, Pravettoni E, Matteoli M. Synaptic and intrinsic mechanisms shape synchronous oscillations in hippocampal neurons in culture. Eur J Neurosci. 1999;11:389–397. - PubMed
-
- Banker GA, Cowan WM. Rat hippocampal neurons in dispersed cell culture. Brain Res. 1977;126:397–342. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
