p25/cyclin-dependent kinase 5 induces production and intraneuronal accumulation of amyloid beta in vivo
- PMID: 17035538
- PMCID: PMC6674706
- DOI: 10.1523/JNEUROSCI.3133-06.2006
p25/cyclin-dependent kinase 5 induces production and intraneuronal accumulation of amyloid beta in vivo
Abstract
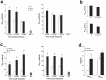
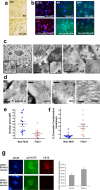
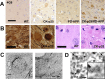
Aberrant processing of the amyloid precursor protein (APP) and the subsequent accumulation of amyloid beta (Abeta) peptide has been widely established as a central event in Alzheimer's disease (AD) pathogenesis. The sequential cleavage steps required for the generation of Abeta are well outlined; however, there is a relative dearth of knowledge pertaining to signaling pathways and molecular mechanisms that can modulate this process. Here, we demonstrate a novel role for p25/cyclin-dependent kinase 5 (Cdk5) in regulating APP processing, Abeta peptide generation, and intraneuronal Abeta accumulation in inducible p25 transgenic and compound PD-APP transgenic mouse models that demonstrate deregulated Cdk5 activity and a neurodegenerative phenotype. Induction of p25 resulted in enhanced forebrain Abeta levels before any evidence of neuropathology in these mice. Intracellular Abeta accumulated in perinuclear regions and distended axons within the forebrains of these mice. Evidence for modulations in axonal transport or beta-site APP cleaving enzyme 1 protein levels and activity are presented as mechanisms that may account for the Abeta accumulation caused by p25/Cdk5 deregulation. Collectively, these findings delineate a novel pathological mechanism involving aberrant APP processing by p25/Cdk5 and have important implications in AD pathogenesis.
Figures

References
-
- Beglopoulos V, Sun X, Saura CA, Lemere CA, Kim RD, Shen J. Reduced beta-amyloid production and increased inflammatory responses in presenilin conditional knock-out mice. J Biol Chem. 2004;279:46907–46914. - PubMed
-
- Billings LM, Oddo S, Green KN, McGaugh JL, Laferla FM. Intraneuronal Abeta causes the onset of early Alzheimer's disease-related cognitive deficits in transgenic mice. Neuron. 2005;45:675–688. - PubMed
-
- Casas C, Sergeant N, Itier JM, Blanchard V, Wirths O, van der Kolk N, Vingtdeux V, van de Steeg E, Ret G, Canton T, Drobecq H, Clark A, Bonici B, Delacourte A, Benavides J, Schmitz C, Tremp G, Bayer TA, Benoit P, Pradier L. Massive CA1/2 neuronal loss with intraneuronal and N-terminal truncated Abeta42 accumulation in a novel Alzheimer transgenic model. Am J Pathol. 2004;165:1289–1300. - PMC - PubMed
-
- Cruz JC, Tseng HC, Goldman JA, Shih H, Tsai LH. Aberrant Cdk5 activation by p25 triggers pathological events leading to neurodegeneration and neurofibrillary tangles. Neuron. 2003;40:471–483. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
