Alpha-synuclein expression modulates microglial activation phenotype
- PMID: 17035541
- PMCID: PMC6674709
- DOI: 10.1523/JNEUROSCI.1799-06.2006
Alpha-synuclein expression modulates microglial activation phenotype
Abstract
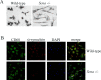
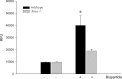
Recent Parkinson's disease research has focused on understanding the function of the cytosolic protein, alpha-synuclein, and its contribution to disease mechanisms. Within neurons, alpha-synuclein is hypothesized to have a role in regulating synaptic plasticity, vesicle release, and trafficking. In contrast, glial-expressed alpha-synuclein remains poorly described. Here, we examine the consequence of a loss of alpha-synuclein expression on microglial activation. Using a postnatal brain-derived culture system, we defined the phenotype of microglia from wild-type and knock-out alpha-synuclein mice (Scna-/-). Scna-/- microglia displayed a basally increased reactive phenotype compared with the wild-type cells and an exacerbated reactive phenotype after stimulation. They also exhibited dramatic morphologic differences compared with wild-type, presenting as large, ramified cells filled with vacuole-like structures. This corresponded with increased protein levels of activation markers, CD68 and beta1 integrin, in the Scna-/- cells. More importantly, Scna-/- microglia, after stimulation, secreted elevated levels of proinflammatory cytokines, TNFalpha (tumor necrosis factor alpha) and IL-6 (interleukin-6), compared with wild type. However, despite the reactive phenotype, Scna-/- cells had impaired phagocytic ability. We demonstrate for the first time that alpha-synuclein plays a critical role in modulating microglial activation state. We suggest that altered microglial alpha-synuclein expression will affect their phenotype as has already been demonstrated in neurons. This has direct ramifications for the contribution of microglia to the pathophysiology of disease, particularly in familial cases linked to altered alpha-synuclein expression.
Figures


References
-
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho W-H, Castillo PE, Shinsky N, Verdugo JMG, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lacking α-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. - PubMed
-
- Ahn BH, Rhim H, Kim SY, Sung YM, Lee MY, Choi JY, Wolozin B, Chang JS, Lee YH, Kwon TK, Chung KC, Yoon SH, Hahn SJ, Kim MS, Jo YH, Min do S. Alpha-synuclein interacts with phospholipase D isozymes and inhibits pervanadate-induced phospholipase D activation in human embryonic kidney-293 cells. J Biol Chem. 2002;277:12334–12342. - PubMed
-
- Boka G, Anglade P, Wallach D, Javoy-Agid F, Agid Y, Hirsch EC. Immunocytochemical analysis of tumor necrosis factor and its receptors in Parkinson's disease. Neurosci Lett. 1994;172:151–154. - PubMed
-
- Cabin DE, Shimazu K, Murphy D, Cole NB, Gottschalk W, McIlwain KL, Orrison B, Chen A, Ellis CE, Paylor R, Lu B, Nussbaum RL. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking α-synuclein. J Neurosci. 2002;22:8797–8807. - PMC - PubMed
-
- Castagnet PI, Golovko MY, Barcelo-Coblijn GC, Nussbaum RL, Murphy EJ. Fatty acid incorporation is decreased in astrocytes cultured from alpha-synuclein gene-ablated mice. J Neurochem. 2005;94:839–849. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
