Dopaminergic control of sleep-wake states
- PMID: 17035544
- PMCID: PMC6674686
- DOI: 10.1523/JNEUROSCI.1767-06.2006
Dopaminergic control of sleep-wake states
Abstract
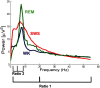
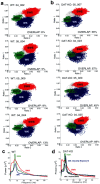
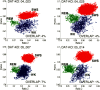
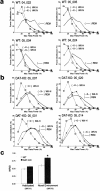
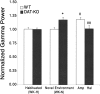
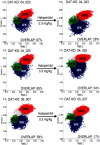
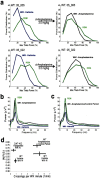
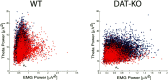
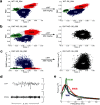
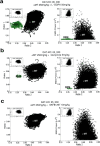
Dopamine depletion is involved in the pathophysiology of Parkinson's disease, whereas hyperdopaminergia may play a fundamental role in generating endophenotypes associated with schizophrenia. Sleep disturbances are known to occur in both schizophrenia and Parkinson's disease, suggesting that dopamine plays a role in regulating the sleep-wake cycle. Here, we show that novelty-exposed hyperdopaminergic mice enter a novel awake state characterized by spectral patterns of hippocampal local field potentials that resemble electrophysiological activity observed during rapid-eye-movement (REM) sleep. Treatment with haloperidol, a D2 dopamine receptor antagonist, reduces this abnormal intrusion of REM-like activity during wakefulness. Conversely, mice acutely depleted of dopamine enter a different novel awake state characterized by spectral patterns of hippocampal local field potentials that resemble electrophysiological activity observed during slow-wave sleep (SWS). This dopamine-depleted state is marked by an apparent suppression of SWS and a complete suppression of REM sleep. Treatment with D2 (but not D1) dopamine receptor agonists recovers REM sleep in these mice. Altogether, these results indicate that dopamine regulates the generation of sleep-wake states. We propose that psychosis and the sleep disturbances experienced by Parkinsonian patients result from dopamine-mediated disturbances of REM sleep.
Figures



References
-
- Abbott A. Neuroscience: while you were sleeping. Nature. 2005;437:1220–1222. - PubMed
-
- Adler CH. Nonmotor complications in Parkinson's disease. Mov Disord. 2005;20:S23–S29. - PubMed
-
- Aserinsky E, Kleitman N. Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science. 1953;118:273–274. - PubMed
-
- Bjorklund A, Lindvall O. Dopamine-containing systems in the CNS. In: Hokfelt T, editor. Handbook of chemical neuroanatomy. Amsterdam: Elsevier; 1984. pp. 52–122.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
