In vivo phosphorylation of CFTR promotes formation of a nucleotide-binding domain heterodimer
- PMID: 17036051
- PMCID: PMC1618097
- DOI: 10.1038/sj.emboj.7601373
In vivo phosphorylation of CFTR promotes formation of a nucleotide-binding domain heterodimer
Abstract
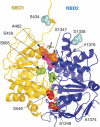
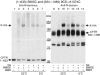
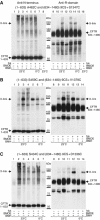
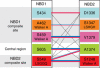
The human ATP-binding cassette (ABC) protein CFTR (cystic fibrosis transmembrane conductance regulator) is a chloride channel, whose dysfunction causes cystic fibrosis. To gain structural insight into the dynamic interaction between CFTR's nucleotide-binding domains (NBDs) proposed to underlie channel gating, we introduced target cysteines into the NBDs, expressed the channels in Xenopus oocytes, and used in vivo sulfhydryl-specific crosslinking to directly examine the cysteines' proximity. We tested five cysteine pairs, each comprising one introduced cysteine in the NH(2)-terminal NBD1 and another in the COOH-terminal NBD2. Identification of crosslinked product was facilitated by co-expression of NH(2)-terminal and COOH-terminal CFTR half channels each containing one NBD. The COOH-terminal half channel lacked all native cysteines. None of CFTR's 18 native cysteines was found essential for wild type-like, phosphorylation- and ATP-dependent, channel gating. The observed crosslinks demonstrate that NBD1 and NBD2 interact in a head-to-tail configuration analogous to that in homodimeric crystal structures of nucleotide-bound prokaryotic NBDs. CFTR phosphorylation by PKA strongly promoted both crosslinking and opening of the split channels, firmly linking head-to-tail NBD1-NBD2 association to channel opening.
Figures







References
-
- Aleksandrov L, Aleksandrov AA, Chang XB, Riordan JR (2002) The first nucleotide binding domain of cystic fibrosis transmembrane conductance regulator is a site of stable nucleotide interaction, whereas the second is a site of rapid turnover. J Biol Chem 277: 15419–15425 - PubMed
-
- Armstrong S, Tabernero L, Zhang H, Hermodson M, Stauffacher C (1998) Powering the ABC transporters: the 2.5 Å crystal structure of the ABC domain of RBSA. Pediatr Pulm 17: 91–92
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

