In vivo measurement of plaque burden in a mouse model of Alzheimer's disease
- PMID: 17036339
- PMCID: PMC2859034
- DOI: 10.1002/jmri.20751
In vivo measurement of plaque burden in a mouse model of Alzheimer's disease
Abstract
Purpose: To demonstrate an MRI method for directly visualizing amyloid-beta (Abeta) plaques in the APP/PS1 transgenic (tg) mouse brain in vivo, and show that T1rho relaxation rate increases progressively with Alzheimer's disease (AD)-related pathology in the tg mouse brain.
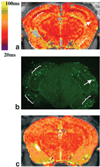
Materials and methods: We obtained in vivo MR images of a mouse model of AD (APP/PS1) that overexpresses human amyloid precursor protein, and measured T1rho via quantitative relaxometric maps.
Results: A significant decrease in T1rho was observed in the cortex and hippocampus of 12- and 18-month-old animals compared to their age-matched controls. There was also a correlation between changes in T1rho and the age of the animals.
Conclusion: T1rho relaxometry may be a sensitive method for noninvasively determining AD-related pathology in APP/PS1 mice.
Copyright (c) 2006 Wiley-Liss, Inc.
Figures



References
-
- Dawbarn D, Allen SJ. Neurobiology of Alzheimer’s disease. New York: Oxford University Press; 2001. p. 409. xvi.
-
- Prince M. The need for research on dementia in developing countries. Trop Med Int Health. 1997;2:993–1000. - PubMed
-
- Selkoe DJ. Translating cell biology into therapeutic advances in Alzheimer’s disease. Nature. 1999;399 Suppl 6738:A23–A31. - PubMed
-
- Lee VM, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121–1159. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

