Distinct patterns of mucosal apoptosis in H pylori-associated gastric ulcer are associated with altered FasL and perforin cytotoxic pathways
- PMID: 17036384
- PMCID: PMC4088106
- DOI: 10.3748/wjg.v12.i38.6133
Distinct patterns of mucosal apoptosis in H pylori-associated gastric ulcer are associated with altered FasL and perforin cytotoxic pathways
Abstract
Aim: To analyze the level of apoptosis in different mucosal compartments and the differential expression of Fas/Fas-ligand and perforin in H pylori-associated gastric ulcer.
Methods: Antral specimens from patients with H pylori-related active gastric ulcer (GU), H pylori-related gastritis, and non-infected controls were analysed for densities and distribution of apoptotic cells determined by the TdT-mediated dUDP-biotin nick-end-labelling method. GU patients were submitted to eradication therapy with follow-up biopsy after 60 d. Fas, FasL, and perforin-expressing cells were assessed by immunoperoxidase, and with anti-CD3, anti-CD20 and anti-CD68 by double immunofluorescence and confocal microscopy. Quantitative analysis was performed using a computer-assisted image analyser.
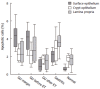
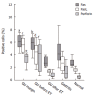
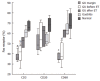
Results: H pylori-infected antrum showed greater surface epithelial apoptosis which decreased after eradication therapy. In the lamina propria, higher rates of mononuclear cell apoptosis were observed in H pylori-gastritis. Co-expression of Fas with T-cell and macrophage markers was reduced in GU. FasL- and perforin-expressing cells were increased in H pylori-infection and correlated with epithelial apoptosis. Perforin-expressing cells were also increased in GU compared with H pylori-gastritis.
Conclusion: Epithelial apoptosis is increased in H pylori-infection and correlates to FasL- and perforin-expression by T cells. Expression of perforin is correlated with the tissue damage, and may represent the enhancement of a distinct cytotoxic pathway in GU. Increased expression of FasL not paralleled by Fas on T-cells and macrophages may indicate a reduced susceptibility to the Fas/FasL-mediated apoptosis of lymphoid cells in H pylori-infection.
Figures



Similar articles
-
Apoptosis in the intestinal mucosa of patients with inflammatory bowel disease: evidence of altered expression of FasL and perforin cytotoxic pathways.Int J Colorectal Dis. 2005 May;20(3):277-86. doi: 10.1007/s00384-004-0639-8. Epub 2004 Oct 22. Int J Colorectal Dis. 2005. PMID: 15503066
-
Upregulation of mucosal soluble fas ligand and interferon-gamma may be involved in ulcerogenesis in patients with Helicobacter pylori-positive gastric ulcer.Scand J Gastroenterol. 2002 May;37(5):501-11. doi: 10.1080/00365520252903026. Scand J Gastroenterol. 2002. PMID: 12059049
-
Perforin and granzyme B of cytotoxic T lymphocyte mediate apoptosis irrespective of Helicobacter pylori infection: possible act as a trigger of peptic ulcer formation.Hepatogastroenterology. 2003 Nov-Dec;50(54):1774-9. Hepatogastroenterology. 2003. PMID: 14696402
-
Modulation of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis by Helicobacter pylori in immune pathogenesis of gastric mucosal damage.J Microbiol Immunol Infect. 2017 Feb;50(1):4-9. doi: 10.1016/j.jmii.2016.01.002. Epub 2016 Jan 29. J Microbiol Immunol Infect. 2017. PMID: 26947589 Review.
-
Immune effector mechanisms in myocardial pathologies.Int J Mol Med. 2000 Jul;6(1):3-16. doi: 10.3892/ijmm.6.1.3. Int J Mol Med. 2000. PMID: 10851260 Review.
References
-
- Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–1315. - PubMed
-
- Graham DY, Lew GM, Evans DG Jr, Evans DJ, Klein PD. Effect of triple therapy (antibiotics plus bismuth) on duodenal ulcer healing. A randomized controlled trial. Ann Intern Med. 1991;115:266–269. - PubMed
-
- Parsonnet J, Hansen S, Rodriguez L, Gelb AB, Warnke RA, Jellum E, Orentreich N, Vogelman JH, Friedman GD. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994;330:1267–1271. - PubMed
-
- Blaser MJ, Perez-Perez GI, Kleanthous H, Cover TL, Peek RM, Chyou PH, Stemmermann GN, Nomura A. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55:2111–2115. - PubMed
-
- Ernst P. Review article: the role of inflammation in the pathogenesis of gastric cancer. Aliment Pharmacol Ther. 1999;13 Suppl 1:13–18. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

