An herbal formula, CGX, exerts hepatotherapeutic effects on dimethylnitrosamine-induced chronic liver injury model in rats
- PMID: 17036385
- PMCID: PMC4088107
- DOI: 10.3748/wjg.v12.i38.6142
An herbal formula, CGX, exerts hepatotherapeutic effects on dimethylnitrosamine-induced chronic liver injury model in rats
Abstract
Aim: To evaluate the therapeutic effect of Chunggan extract (CGX), a modified traditional Chinese hepatotherapeutic herbal, on the dimethylnitrosamine (DMN)-induced chronic liver injury model in rats.
Methods: Liver injuries were induced in Wistar rats by injection of DMN (ip, 10 mg/mL per kg) for 3 consecutive days per week for 4 wk. The rats were administered with CGX (po, 100 or 200 mg/kg per day) or distilled water as a control daily for 4 wk starting from the 15(th) d of the DMN treatment. Biochemical parameters (serum albumin, bilirubin, ALP, AST and ALT), lipid peroxides, hydroxyproline, as well as histological changes in liver tissues were analyzed. In addition, gene expression of TNF-alpha, TGF-beta, TIMP-1, TIMP-2, PDGF-beta, and MMP-2, all of which are known to be associated with liver fibrosis, were analyzed using real-time PCR.
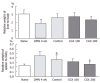
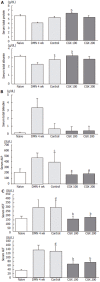
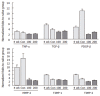
Results: CGX administration restored the spleen weight to normal after having been increased by DMN treatment. Biochemical analysis of the serum demonstrated that CGX significantly decreased the serum level of ALP (P < 0.05), ALT (P < 0.01), and AST (P < 0.01) that had been elevated by DMN treatment. CGX administration moderately lowered lipid peroxide production and markedly lowered hydroxyproline generation caused by DMN treatment in accordance with histopathological examination. DMN treatment induced a highly up-regulated expression of TNF-alpha, TGF-beta, TIMP-1, TIMP-2, PDGF-beta, and MMP-2. Of these, the gene expression encoding PDGF-beta and MMP-2 was still further enhanced 2 wk after secession of the 4-wk DMN treatment, and was remarkably ameliorated by CGX administration.
Conclusion: CGX exhibits hepatotherapeutic proper-ties against chronic hepatocellular destruction and consequential liver fibrosis.
Figures



Similar articles
-
Chinese medicine CGA formula ameliorates DMN-induced liver fibrosis in rats via inhibiting MMP2/9, TIMP1/2 and the TGF-β/Smad signaling pathways.Acta Pharmacol Sin. 2016 Jun;37(6):783-93. doi: 10.1038/aps.2016.35. Epub 2016 May 2. Acta Pharmacol Sin. 2016. PMID: 27133300 Free PMC article.
-
[Effects of Chinese herbal medicine Xiaopi Pill in preventing rats from dimethylnitrosamine-induced liver fibrosis].Zhong Xi Yi Jie He Xue Bao. 2012 Nov;10(11):1286-92. doi: 10.3736/jcim20121113. Zhong Xi Yi Jie He Xue Bao. 2012. PMID: 23158948 Chinese.
-
A traditional formula, Chunggan extract, attenuates thioacetamide-induced hepatofibrosis via GSH system in rats.Hum Exp Toxicol. 2011 Sep;30(9):1322-32. doi: 10.1177/0960327110389502. Epub 2010 Nov 11. Hum Exp Toxicol. 2011. PMID: 21071552
-
Antifibrotic effects of CGX, a traditional herbal formula, and its mechanisms in rats.J Ethnopharmacol. 2010 Feb 3;127(2):534-42. doi: 10.1016/j.jep.2009.10.001. Epub 2009 Oct 13. J Ethnopharmacol. 2010. PMID: 19833189
-
[Formula-syndrome correlation study of three classical anti-jaundice formulas in inhibition of liver fibrosis induced by dimethylnitrosamine in rats].Zhong Xi Yi Jie He Xue Bao. 2012 Dec;10(12):1405-12. doi: 10.3736/jcim20121212. Zhong Xi Yi Jie He Xue Bao. 2012. PMID: 23257134 Chinese.
Cited by
-
Serum Metabolomic Characterization of Liver Fibrosis in Rats and Anti-Fibrotic Effects of Yin-Chen-Hao-Tang.Molecules. 2016 Jan 21;21(1):E126. doi: 10.3390/molecules21010126. Molecules. 2016. PMID: 26805802 Free PMC article.
-
Protective effects of Flos lonicera extract on acute liver injury by dimethylnitrosamine-induced in rats.J Nat Med. 2010 Jul;64(3):288-94. doi: 10.1007/s11418-010-0405-x. Epub 2010 Mar 20. J Nat Med. 2010. PMID: 20306146
-
Ethyl Acetate Fraction of Amomum xanthioides Exerts Antihepatofibrotic Actions via the Regulation of Fibrogenic Cytokines in a Dimethylnitrosamine-Induced Rat Model.Evid Based Complement Alternat Med. 2016;2016:6014380. doi: 10.1155/2016/6014380. Epub 2016 Aug 10. Evid Based Complement Alternat Med. 2016. PMID: 27594891 Free PMC article.
-
Recovery from hepatitis A after Korean medicine-based treatment : a case report.Integr Med Res. 2019 Dec;8(4):257-260. doi: 10.1016/j.imr.2019.11.001. Epub 2019 Nov 7. Integr Med Res. 2019. PMID: 31768311 Free PMC article.
-
Chunggan extract, a traditional herbal formula, ameliorated alcohol-induced hepatic injury in rat model.World J Gastroenterol. 2014 Nov 14;20(42):15703-14. doi: 10.3748/wjg.v20.i42.15703. World J Gastroenterol. 2014. PMID: 25400454 Free PMC article.
References
-
- Rockey DC. Antifibrotic therapy in chronic liver disease. Clin Gastroenterol Hepatol. 2005;3:95–107. - PubMed
-
- Breitkopf K, Haas S, Wiercinska E, Singer MV, Dooley S. Anti-TGF-beta strategies for the treatment of chronic liver disease. Alcohol Clin Exp Res. 2005;29:121S–131S. - PubMed
-
- Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97–107. - PubMed
-
- Kitamura Y, Ninomiya H. Smad expression of hepatic stellate cells in liver cirrhosis in vivo and hepatic stellate cell line in vitro. Pathol Int. 2003;53:18–26. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

