Genetically encoded Ca2+ indicators: using genetics and molecular design to understand complex physiology
- PMID: 17038427
- PMCID: PMC2075121
- DOI: 10.1113/jphysiol.2006.120212
Genetically encoded Ca2+ indicators: using genetics and molecular design to understand complex physiology
Abstract
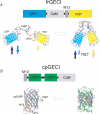
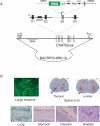
This article reviews genetically encoded Ca2+ indicators (GECIs), with a focus on the use of these novel molecules in the context of understanding complex cell signalling in mammals, in vivo. The review focuses on the advantages and limitations of specific GECI design strategies and the results of experiments in which these molecules have been expressed in transgenic mice, concentrating particularly on recent experiments from our laboratory in which physiological signalling could be monitored in vivo. Finally, newer strategies for effective genetic specification of GECIs are briefly reviewed.
Figures


References
-
- Adams SR, Harootunian AT, Buechler YJ, Taylor SS, Tsien RY. Fluorescence ratio imaging of cyclic AMP in single cells. Nature. 1991;349:694–697. - PubMed
-
- Bacskai BJ, Hochner B, Mahaut-Smith M, Adams SR, Kaang BK, Kandel ER, Tsien RY. Spatially resolved dynamics of cAMP and protein kinase A subunits in Aplysia sensory neurons. Science. 1993;260:222–226. - PubMed
-
- Baker LC, London B, Choi BR, Koren G, Salama G. Enhanced dispersion of repolarization and refractoriness in transgenic mouse hearts promotes reentrant ventricular tachycardia. Circ Res. 2000;86:396–407. - PubMed
-
- Benaim G, Villalobo A. Phosphorylation of calmodulin. Functional implications. Eur J Biochem. 2002;269:3619–3631. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

