Mechanosensitive currents in the neurites of cultured mouse sensory neurones
- PMID: 17038434
- PMCID: PMC1804210
- DOI: 10.1113/jphysiol.2006.117648
Mechanosensitive currents in the neurites of cultured mouse sensory neurones
Abstract
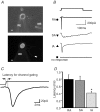
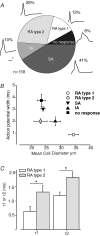
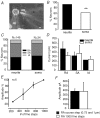
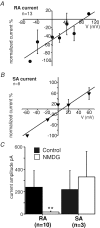
Almost all sensory neurones in the dorsal root ganglia have a mechanosensory function. The transduction of mechanical stimuli in vivo takes place exclusively at the sensory ending. For cutaneous sensory receptors it has so far proved impossible to directly record the mechanically gated receptor potential because of the small size and inaccessibility of the sensory ending. Here we investigate whether mechanosensitive currents are present in the neurites of freshly isolated adult mouse sensory neurones in culture. Almost all sensory neurone neurites possess currents gated by submicrometre displacement stimuli (92%). Three types of mechanically activated conductance were characterized based on different inactivation kinetics. A rapidly adapting conductance was found in larger sensory neurones with narrow action potentials characteristic of mechanoreceptors. Slowly and intermediate adapting conductances were found exclusively in putative nociceptive neurones. Mechanically activated currents with similar kinetics were found also after stimulating the cell soma. However, soma currents were only observed in around 60% of cells tested and the displacement threshold was several times larger than for the neurite (approximately 6 microm). The reversal potential of the rapidly adapting current indicated that this current is largely selective for sodium ions whereas the slowly adapting current is non-selective. It is likely that distinct ion channel entities underlie these two currents. In summary, our data suggest that the high sensitivity and robustness of mechanically gated currents in the sensory neurite make this a useful in vitro model for the mechanosensitive sensory endings in vivo.
Figures


Similar articles
-
Acid-sensing ion channels ASIC2 and ASIC3 do not contribute to mechanically activated currents in mammalian sensory neurones.J Physiol. 2004 May 1;556(Pt 3):691-710. doi: 10.1113/jphysiol.2003.058693. Epub 2004 Feb 27. J Physiol. 2004. PMID: 14990679 Free PMC article.
-
Voltage- and use-dependent inhibition of Na+ channels in rat sensory neurones by 4030W92, a new antihyperalgesic agent.Br J Pharmacol. 1998 Jul;124(5):953-63. doi: 10.1038/sj.bjp.0701919. Br J Pharmacol. 1998. PMID: 9692781 Free PMC article.
-
A novel mechanosensitive channel identified in sensory neurons.Eur J Neurosci. 2006 May;23(10):2543-50. doi: 10.1111/j.1460-9568.2006.04802.x. Eur J Neurosci. 2006. PMID: 16817857
-
[Anatomic and functional identification of sensory neurons in neurophysiologic studies].Postepy Hig Med Dosw. 2000;54(3):351-61. Postepy Hig Med Dosw. 2000. PMID: 10941269 Review. Polish.
-
Biophysical studies of mechanoreceptors.J Appl Physiol (1985). 1986 Apr;60(4):1107-15. doi: 10.1152/jappl.1986.60.4.1107. J Appl Physiol (1985). 1986. PMID: 2422151 Review.
Cited by
-
En masse in vitro functional profiling of the axonal mechanosensitivity of sensory neurons.Proc Natl Acad Sci U S A. 2010 Sep 14;107(37):16336-41. doi: 10.1073/pnas.0914705107. Epub 2010 Aug 24. Proc Natl Acad Sci U S A. 2010. PMID: 20736349 Free PMC article.
-
Piezo2 in Cutaneous and Proprioceptive Mechanotransduction in Vertebrates.Curr Top Membr. 2017;79:197-217. doi: 10.1016/bs.ctm.2016.11.002. Epub 2017 Jan 16. Curr Top Membr. 2017. PMID: 28728817 Free PMC article. Review.
-
Isolated nociceptors reveal multiple specializations for generating irregular ongoing activity associated with ongoing pain.Pain. 2018 Nov;159(11):2347-2362. doi: 10.1097/j.pain.0000000000001341. Pain. 2018. PMID: 30015712 Free PMC article.
-
Investigating Mechanically Activated Currents from Trigeminal Neurons of Nonhuman Primates.eNeuro. 2025 May 12;12(5):ENEURO.0054-25.2025. doi: 10.1523/ENEURO.0054-25.2025. Print 2025 May. eNeuro. 2025. PMID: 40280765 Free PMC article.
-
Sensory Schwann cells set perceptual thresholds for touch and selectively regulate mechanical nociception.Nat Commun. 2024 Feb 6;15(1):898. doi: 10.1038/s41467-024-44845-8. Nat Commun. 2024. PMID: 38320986 Free PMC article.
References
-
- Akopian AN, Sivilotti L, Wood JN. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature. 1996;379:257–262. - PubMed
-
- Cho H, Koo JY, Kim S, Park SP, Yang Y, Oh U. A novel mechanosensitive channel identified in sensory neurons. Eur J Neurosci. 2006;23:2543–2550. - PubMed
-
- Clapham DE, Julius D, Montell C, Schultz G. International Union of Pharmacology. XLIX. Nomenclature and structure-function relationships of transient receptor potential channels. Pharmacol Rev. 2005;57:427–450. - PubMed
-
- Cunningham JT, Wachtel RE, Abboud FM. Mechanosensitive currents in putative aortic baroreceptor neurons in vitro. J Neurophysiol. 1995;73:2094–2098. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

