Enhanced surfactant adsorption via polymer depletion forces: a simple model for reversing surfactant inhibition in acute respiratory distress syndrome
- PMID: 17040987
- PMCID: PMC1697872
- DOI: 10.1529/biophysj.106.091157
Enhanced surfactant adsorption via polymer depletion forces: a simple model for reversing surfactant inhibition in acute respiratory distress syndrome
Abstract
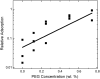
Lung surfactant adsorption to an air-water interface is strongly inhibited by an energy barrier imposed by the competitive adsorption of albumin and other surface-active serum proteins that are present in the lung during acute respiratory distress syndrome. This reduction in surfactant adsorption results in an increased surface tension in the lung and an increase in the work of breathing. The reduction in surfactant adsorption is quantitatively described using a variation of the classical Smolukowski analysis of colloid stability. Albumin adsorbed to the interface induces an energy barrier to surfactant diffusion of order 5 k(B)T, leading to a reduction in adsorption equivalent to reducing the surfactant concentration by a factor of 100. Adding hydrophilic, nonadsorbing polymers such as polyethylene glycol to the subphase provides a depletion attraction between the surfactant aggregates and the interface that eliminates the energy barrier. Surfactant adsorption increases exponentially with polymer concentration as predicted by the simple Asakura and Oosawa model of depletion attraction. Depletion forces can likely be used to overcome barriers to adsorption at a variety of liquid-vapor and solid-liquid interfaces.
Figures


References
-
- Clements, J. A., and M. E. Avery. 1998. Lung surfactant and neonatal respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 157:59–66. - PubMed
-
- Notter, R. H. 2000. Lung Surfactant: Basic Science and Clinical Applications. Marcel Dekker, New York.
-
- Lipp, M. M., K. Y. C. Lee, J. A. Zasadzinski, and A. J. Waring. 1996. Phase and morphology changes in lipid monolayers induced by SP-B protein and its amino-terminal peptide. Science. 273:1196–1199. - PubMed
-
- Haitsma, J. J., P. J. Papadakos, and B. Lachmann. 2004. Surfactant therapy for acute lung injury/acute respiratory distress syndrome. Curr. Opin. Crit. Care. 10:18–22. - PubMed
-
- Avery, M. E. 2000. Surfactant deficiency in hyaline membrane disease. Am. J. Respir. Crit. Care Med. 161:1074–1075. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

