Nuclear magnetic resonance spectroscopy-based metabolite profiling of transgenic tomato fruit engineered to accumulate spermidine and spermine reveals enhanced anabolic and nitrogen-carbon interactions
- PMID: 17041034
- PMCID: PMC1676058
- DOI: 10.1104/pp.106.084400
Nuclear magnetic resonance spectroscopy-based metabolite profiling of transgenic tomato fruit engineered to accumulate spermidine and spermine reveals enhanced anabolic and nitrogen-carbon interactions
Abstract
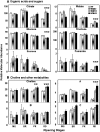
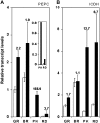
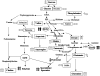
Polyamines are ubiquitous aliphatic amines that have been implicated in myriad processes, but their precise biochemical roles are not fully understood. We have carried out metabolite profiling analyses of transgenic tomato (Solanum lycopersicum) fruit engineered to accumulate the higher polyamines spermidine (Spd) and spermine (Spm) to bring an insight into the metabolic processes that Spd/Spm regulate in plants. NMR spectroscopic analysis revealed distinct metabolite trends in the transgenic and wild-type/azygous fruits ripened off the vine. Distinct metabolites (glutamine, asparagine, choline, citrate, fumarate, malate, and an unidentified compound A) accumulated in the red transgenic fruit, while the levels of valine, aspartic acid, sucrose, and glucose were significantly lower as compared to the control (wild-type and azygous) red fruit. The levels of isoleucine, glucose, gamma-aminobutyrate, phenylalanine, and fructose remained similar in the nontransgenic and transgenic fruits. Statistical treatment of the metabolite variables distinguished the control fruits from the transgenic fruit and provided credence to the pronounced, differential metabolite profiles seen during ripening of the transgenic fruits. The pathways involved in the nitrogen sensing/signaling and carbon metabolism seem preferentially activated in the high Spd/Spm transgenics. The metabolite profiling analysis suggests that Spd and Spm are perceived as nitrogenous metabolites by the fruit cells, which in turn results in the stimulation of carbon sequestration. This is seen manifested in higher respiratory activity and up-regulation of phosphoenolpyruvate carboxylase and NADP-dependent isocitrate dehydrogenase transcripts in the transgenic fruit compared to controls, indicating high metabolic status of the transgenics even late in fruit ripening.
Figures





References
-
- Abeles FB, Morgan PW, Saltveit ME Jr (1992) Ethylene in Plant Biology. Academic Press, New York
-
- Amato ME, Ansanelli G, Fisichella S, Lamanna R, Scarlata G, Sobolev AP, Segre AL (2004) Wheat flour enzymatic amylolysis monitored by in situ 1H-NMR spectroscopy. J Agric Food Chem 52: 823–831 - PubMed
-
- Bauer GA, Bazzaz FA, Minocha R, Long S, Magill A, Aber J, Berntson GM (2004) Effects of chronic N additions on tissue chemistry, photosynthetic capacity, and carbon sequestration potential of a red pine (Pinus resinosa Ait.) stand in NE United States. For Ecol Manage 196: 173–186
-
- Biale JB, Young RE (1981) Respiration and ripening in fruits: retrospect and prospect. In J Friend, MJC Rhodes, eds, Recent Advances in the Biochemistry of Fruits and Vegetables. Academic Press, New York, pp 103–109
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

