Staphylococcus aureus IsdB is a hemoglobin receptor required for heme iron utilization
- PMID: 17041042
- PMCID: PMC1698231
- DOI: 10.1128/JB.01335-06
Staphylococcus aureus IsdB is a hemoglobin receptor required for heme iron utilization
Abstract
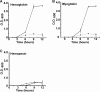
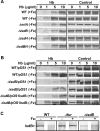
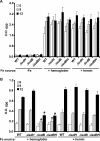
The pathogenesis of human infections caused by the gram-positive microbe Staphylococcus aureus has been previously shown to be reliant on the acquisition of iron from host hemoproteins. The iron-regulated surface determinant system (Isd) encodes a heme transport apparatus containing three cell wall-anchored proteins (IsdA, IsdB, and IsdH) that are exposed on the staphylococcal surface and hence have the potential to interact with human hemoproteins. Here we report that S. aureus can utilize the host hemoproteins hemoglobin and myoglobin, but not hemopexin, as iron sources for bacterial growth. We demonstrate that staphylococci capture hemoglobin on the bacterial surface via IsdB and that inactivation of isdB, but not isdA or isdH, significantly decreases hemoglobin binding to the staphylococcal cell wall and impairs the ability of S. aureus to utilize hemoglobin as an iron source. Stable-isotope-tracking experiments revealed removal of heme iron from hemoglobin and transport of this compound into staphylococci. Importantly, mutants lacking isdB, but not isdH, display a reduction in virulence in a murine model of abscess formation. Thus, IsdB-mediated scavenging of iron from hemoglobin represents an important virulence strategy for S. aureus replication in host tissues and for the establishment of persistent staphylococcal infections.
Figures



Similar articles
-
IsdB-dependent hemoglobin binding is required for acquisition of heme by Staphylococcus aureus.J Infect Dis. 2014 Jun 1;209(11):1764-72. doi: 10.1093/infdis/jit817. Epub 2013 Dec 13. J Infect Dis. 2014. PMID: 24338348 Free PMC article.
-
The iron-regulated surface proteins IsdA, IsdB, and IsdH are not required for heme iron utilization in Staphylococcus aureus.FEMS Microbiol Lett. 2012 Apr;329(1):93-100. doi: 10.1111/j.1574-6968.2012.02502.x. Epub 2012 Feb 9. FEMS Microbiol Lett. 2012. PMID: 22268825
-
Subcellular localization of the Staphylococcus aureus heme iron transport components IsdA and IsdB.Infect Immun. 2009 Jul;77(7):2624-34. doi: 10.1128/IAI.01531-08. Epub 2009 Apr 27. Infect Immun. 2009. PMID: 19398548 Free PMC article.
-
Structural biology of heme binding in the Staphylococcus aureus Isd system.J Inorg Biochem. 2010 Mar;104(3):341-8. doi: 10.1016/j.jinorgbio.2009.09.012. Epub 2009 Sep 26. J Inorg Biochem. 2010. PMID: 19853304 Review.
-
Intracellular metalloporphyrin metabolism in Staphylococcus aureus.Biometals. 2007 Jun;20(3-4):333-45. doi: 10.1007/s10534-006-9032-0. Epub 2007 Mar 27. Biometals. 2007. PMID: 17387580 Review.
Cited by
-
The NEAT Domain-Containing Proteins of Clostridium perfringens Bind Heme.PLoS One. 2016 Sep 16;11(9):e0162981. doi: 10.1371/journal.pone.0162981. eCollection 2016. PLoS One. 2016. PMID: 27637108 Free PMC article.
-
A battle for iron: host sequestration and Staphylococcus aureus acquisition.Microbes Infect. 2012 Mar;14(3):217-27. doi: 10.1016/j.micinf.2011.11.001. Epub 2011 Nov 15. Microbes Infect. 2012. PMID: 22123296 Free PMC article. Review.
-
Staphylococcus aureus uses a novel multidomain receptor to break apart human hemoglobin and steal its heme.J Biol Chem. 2013 Jan 11;288(2):1065-78. doi: 10.1074/jbc.M112.419119. Epub 2012 Nov 6. J Biol Chem. 2013. PMID: 23132864 Free PMC article.
-
Staphylococcus aureus vaccines: Deviating from the carol.J Exp Med. 2016 Aug 22;213(9):1645-53. doi: 10.1084/jem.20160569. Epub 2016 Aug 15. J Exp Med. 2016. PMID: 27526714 Free PMC article. Review.
-
Molecular Basis for the Evolution of Species-Specific Hemoglobin Capture by Staphylococcus aureus.mBio. 2018 Nov 20;9(6):e01524-18. doi: 10.1128/mBio.01524-18. mBio. 2018. PMID: 30459189 Free PMC article.
References
-
- Bae, T., and O. Schneewind. 2006. Allelic replacement in Staphylococcus aureus with inducible counter-selection. Plasmid 55:58-63. - PubMed
-
- Bierne, H., S. K. Mazmanian, M. Trost, M. G. Pucciarelli, G. Liu, P. Dehoux, L. Jansch, F. Garcia-del Portillo, O. Schneewind, and P. Cossart. 2002. Inactivation of the srtA gene in Listeria monocytogenes inhibits anchoring of surface proteins and affects virulence. Mol. Microbiol. 43:869-881. - PubMed
-
- Bradley, J. S. 2005. Newer antistaphylococcal agents. Curr. Opin. Pediatr. 17:71-77. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

