A pentatricopeptide repeat protein facilitates the trans-splicing of the maize chloroplast rps12 pre-mRNA
- PMID: 17041147
- PMCID: PMC1626628
- DOI: 10.1105/tpc.106.046110
A pentatricopeptide repeat protein facilitates the trans-splicing of the maize chloroplast rps12 pre-mRNA
Abstract
The pentatricopeptide repeat (PPR) is a degenerate 35-amino acid repeat motif that is widely distributed among eukaryotes. Genetic, biochemical, and bioinformatic data suggest that many PPR proteins influence specific posttranscriptional steps in mitochondrial or chloroplast gene expression and that they may typically bind RNA. However, biological functions have been determined for only a few PPR proteins, and with few exceptions, substrate RNAs are unknown. To gain insight into the functions and substrates of the PPR protein family, we characterized the maize (Zea mays) nuclear gene ppr4, which encodes a chloroplast-targeted protein harboring both a PPR tract and an RNA recognition motif. Microarray analysis of RNA that coimmunoprecipitates with PPR4 showed that PPR4 is associated in vivo with the first intron of the plastid rps12 pre-mRNA, a group II intron that is transcribed in segments and spliced in trans. ppr4 mutants were recovered through a reverse-genetic screen and shown to be defective for rps12 trans-splicing. The observations that PPR4 is associated in vivo with rps12-intron 1 and that it is also required for its splicing demonstrate that PPR4 is an rps12 trans-splicing factor. These findings add trans-splicing to the list of RNA-related functions associated with PPR proteins and suggest that plastid group II trans-splicing is performed by different machineries in vascular plants and algae.
Figures




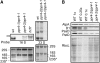


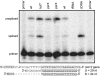
References
-
- Alonso, J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301 653–657. - PubMed
-
- Barkan, A. (1998). Approaches to investigating nuclear genes that function in chloroplast biogenesis in land plants. Methods Enzymol. 297 38–57.
-
- Barkan, A. (2004). Intron splicing in plant organelles. In Molecular Biology and Biotechnology of Plant Organelles, H. Daniell and C. Chase, eds (Dordrecht, The Netherlands: Springer), pp. 295–322.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

