Nonsegmented negative-strand viruses as vaccine vectors
- PMID: 17041210
- PMCID: PMC1641758
- DOI: 10.1128/JVI.00919-06
Nonsegmented negative-strand viruses as vaccine vectors
Figures

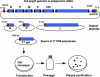

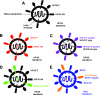
Comment in
-
Caution about Newcastle disease virus-based live attenuated vaccine.J Virol. 2008 Jul;82(13):6782. doi: 10.1128/JVI.00370-08. J Virol. 2008. PMID: 18544791 Free PMC article. No abstract available.
References
-
- Bartlett, E. J., E. Amaro-Carambot, S. R. Surman, J. T. Newman, P. L. Collins, B. R. Murphy, and M. H. Skiadopoulos. 2005. Human parainfluenza virus type I (HPIV1) vaccine candidates designed by reverse genetics are attenuated and efficacious in African green monkeys. Vaccine 23:4631-4646. - PubMed
-
- Buchholz, U. J., S. Biacchesi, Q. N. Pham, K. C. Tran, L. Yang, C. L. Luongo, M. H. Skiadopoulos, B. R. Murphy, and P. L. Collins. 2005. Deletion of M2 gene open reading frames 1 and 2 of human metapneumovirus: effects on RNA synthesis, attenuation, and immunogenicity. J. Virol. 79:6588-6597. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

