Interleukin-10 determines viral clearance or persistence in vivo
- PMID: 17041596
- PMCID: PMC2535582
- DOI: 10.1038/nm1492
Interleukin-10 determines viral clearance or persistence in vivo
Abstract
Persistent viral infections are a major health concern. One obstacle inhibiting the clearance of persistent infections is functional inactivation of antiviral T cells. Although such immunosuppression occurs rapidly after infection, the mechanisms that induce the loss of T-cell activity and promote viral persistence are unknown. Herein we document that persistent viral infection in mice results in a significant upregulation of interleukin (IL)-10 by antigen-presenting cells, leading to impaired T-cell responses. Genetic removal of Il10 resulted in the maintenance of robust effector T-cell responses, the rapid elimination of virus and the development of antiviral memory T-cell responses. Therapeutic administration of an antibody that blocks the IL-10 receptor restored T-cell function and eliminated viral infection. Thus, we identify a single molecule that directly induces immunosuppression leading to viral persistence and demonstrate that a therapy to neutralize IL-10 results in T-cell recovery and the prevention of viral persistence.
Figures



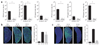
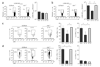

Comment in
-
Virus scores a perfect 10.Nat Med. 2006 Nov;12(11):1246-8. doi: 10.1038/nm1106-1246. Nat Med. 2006. PMID: 17088891 No abstract available.
-
Interleukin-10 and viral clearance: translation to viral hepatitis.Gastroenterology. 2007 Jun;132(7):2611-3. doi: 10.1053/j.gastro.2007.04.049. Gastroenterology. 2007. PMID: 17570238 No abstract available.
References
-
- Rosenberg ES, et al. Immune control of HIV-1 after early treatment of acute infection. Nature. 2000;407:523–526. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

